Effects of Resistance Training and/or Protein Supplementation on Usual Gait Speed in Postmenopausal Women: A Systematic Review and Meta-Analysis
Article information
Trans Abstract
Purpose
The present review aimed to verify the meta-effects of resistance training (RT) or protein supplementation (PS) on usual gait speed (UGS) in postmenopausal women and the additive effect of RT and PS on UGS.
Methods
A systematic literature search of PubMed/Medline and Web of Science (core collection) was performed from inception to December 31, 2021. Electronic search methods were used to identify 18 relevant randomized controlled trials. Meta-analyses of standardized mean difference (SMD) calculated using Hedges’ g between RT and/or PS groups versus control groups were conducted using a random-effects model. The meta-effects are presented in a forest plot with a 95% confidence interval (CI).
Results
The meta-analysis showed that RT significantly improved UGS (SMD, 0.40; 95% CI, 0.12-0.68; p=.006), while PS did not improve UGS (SMD, −0.17; 95% CI, −0.80 to 0.46; p=.601). A subgroup analysis indicated that there were significant increases in UGS after both ‘RT-only intervention (SMD, 0.30; 95% CI, 0.01 to 0.59; p=.046)’ and ‘RT combined with balance training (BT) (SMD, 0.65; 95% CI, 0.17-1.13; p=.008)’, while there was no significant increase in UGS after ‘RT combined with power training (PT) (SMD, −0.08; 95% CI, −0.62 to 0.45; p=.765)’. There was no additive effect on UGS after RT combined with PS (SMD, −0.06; 95% CI, −0.36 to 0.24; p=.699).
Conclusions
This study revealed that RT significantly improved UGS in postmenopausal women, and adding BT to RT further improved UGS. In addition, there was no significant improvement in UGS after PS in postmenopausal women, and no additive effect on UGS after RT combined with PS.
INTRODUCTION
Walking is an essential daily activity for many of us. For this reason, walking ability is a crucial component of an independent and healthy life. Especially, walking speed (gait speed) of a usual pace has important clinical applications as it is an indicator of physical function, indepen-dence, and mobility limitations [1,2]. The decline in usual gait speed (UGS) is associated with adverse outcomes such as falls, immobility, cognitive impairment, dementia, cardiovascular disease, and even mortality [3,4]. UGS tends to decrease with aging because of shorter step length owing to the deterioration in lower-limb muscular strength, range of motion, and balance ability [5]. In particular, lower-limb muscle mass as well as the strength of the lower-limb and trunk muscles are significant predictors of the decline in UGS [6–8]. The cutoff value (<1.0 m/s) of UGS is used as a diagnostic criteria for sarcopenia [9], and a lower value (≤0.8 m/s) has recently been defined as an indicator of severe sarcopenia [10]. Hence, as UGS is profoundly linked to age-related diseases, it seems necessary to establish strategies to prevent the reduction in UGS with aging.
Women tend to experience a rapid decline in their physical function in middle age due to menopause, which is a normal physiological change in a woman's aging process. During the menopausal transition, declines in levels of hormones likes progesterone and estrogen may cause significant physiologic changes. The decline in estrogen production especially has an adverse impact on the musculoskeletal system, thereby causing subsequent decreases in bone mass density, muscle mass, muscular strength, muscular power, and UGS [11–13]. In addition, a four-year lon-gitudinal study found the annual decrease in UGS and grip strength to be faster in women than men, even among the older adults [14]. Natural menopause occurs at middle age between 45 and 55 years, and its mean age of incidence is around 51 years [15]. Therefore, middle-aged and older women may need to prepare more thoroughly than men in the same age range to regulate the deterioration in physical function caused by menopause.
Exercise training is one of the most effective interventions to improve physical function, including UGS. Resistance training (RT) is specifically recommended to be an efficient strategy for promoting physical functions, muscle mass, and muscular strength [16,17]. Meta-analytical evidence has shown a significant effect of progressive RT with high intensity on UGS in older adults, although the addition of endurance and/or balance training (BT) has been inconsequential in producing additional effects of RT [18]. Hence, RT, which has been designed mainly to improve muscular fitness, is likely to be the most suitable intervention for improving UGS, considering the positive correlation between lower-limb muscular strength and UGS [6,19]. However, the effects of RT on UGS have not been fully investigated, and a few contradicting results have reported no effects of RT on UGS [20,21]. Moreover, to the best of our knowledge, few systematic reviews and meta-analyses on the effects of RT on UGS specific to postmenopausal women have been undertak-en; such studies are necessary considering the hormonal changes and functional decline during the menopausal transition in women.
Nutrition has also been identified as a critical factor in the context of age-related frailty. In this regard, protein supplementation (PS) has been suggested as an effective strategy to prevent age-related loss of muscular strength and physical function, including UGS. Muscle hypertrophy can result when the rates of muscle protein synthesis (MPS) exceed muscle protein breakdown, which may be induced by the adequate consumption of essential amino acids (EAA) as well as dietary protein [22]. A recent longitudinal study showed that higher protein intake (≥1.1 g/kg/day) was inversely associated with frailty, including slower UGS, when compared to lower protein intake in older women (<1.1 g/kg/day) [23]. However, protein intake tends to decrease with aging due to an anorexia of aging caused by age-related changes, such as changes in gastrointesti-nal motility, central control of ingestion, and secretion and peripheral action of the appetite-related hormones [24]. A longitudinal study reported a significant decline in a protein intake from baseline (0.95±0.29 g/kg/day) to three-year follow-up (0.87±0.44 g/kg/day) in healthy older females [25], even though a protein intake of 1.5 g/kg/day is recommended for preventing this population from frailty [26,27]. Therefore, additional PS seems to be necessary to prevent the decline in physical function, including UGS, among postmenopausal women. However, to the best of our knowledge, there are few systematic reviews and meta-analyses of the effects of PS on UGS specific to postmenopausal women.
Therefore, the purpose of this meta-analysis was to verify the meta-effects of RT or PS on improvement in UGS in postmenopausal women. Additionally, we investigated whether there was an additive effect of RT and PS on UGS by comparing the effect between RT combined with PS and RT-only intervention. We hypothesized that RT or PS will have significant beneficial effects on UGS. A second hypothesis was that the combination of RT and PS may create an additive effect on UGS. We expect this systematic review and meta-analysis to provide further evidence for preventing a rapid decline in the physical functions of postmenopausal women.
METHODS
This review was reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [28] and National Evidence-based Healthcare Collaborating Agency (NECA) guidance for systematic reviews and meta-analyses [29].
1. Search strategy
We performed a systematic literature search in PubMed/Medline and Web of Science (core collection) from inception up to December 31, 2021; no year restriction was applied to our search strategy. We used the same search terms in both the databases, and included a mix of Medical Subject Headings (MeSH) and free-text words for key concepts linked to the effect of RT and/or PS on UGS in postmenopausal women. Specifi-cally, we used combinations of the following keywords: (protein supplementation OR protein ingestion OR resistance exercise OR resistance training OR strength training OR weight training) AND (gait speed OR walking speed) AND (middle-aged women OR elderly women OR older women OR postmenopausal women). The search results were downloaded and filtered in EndNote software (X9; Clarivate Analytics, New York, USA). In addition, reference lists of included articles were manually searched to identify possibly eligible studies that were not found through the systematic literature search.
2. Inclusion criteria
A systematic review was carried out to identify randomized controlled trials (RCTs) investigating the effect of RT and/or PS on UGS in postmenopausal women. The PICOS model was used to determine the in-clusion criteria [28,29]. The specific criteria of our research were as fol-lows: (1) P (population): postmenopausal women; (2) I (intervention): RT and/or PS (e.g., whey protein, leucine-enriched EAA, and other protein supplements in combination with other nutrients); (3) C (comparators): non-training control groups or same conditions with placebo groups; (4) O (outcome): UGS; (5) S (study design): RCTs. Lastly, all included studies were published in English.
3. Exclusion criteria
First, studies were excluded if they had used a maximal gait speed test instead of a UGS test, because both the methods have a different purpose of testing. While a UGS is indicative of functional states and nu-merous health outcomes, maximal gait speed is indicative of an individ-ual's capabilities in the community [30]. Second, we excluded studies carried out with participants having medical conditions that affect UGS such as lower limb joint replacements, lower limb osteoarthritis, osteo-penia, osteoporosis, fibromyalgia, dementia, and any cancer.
4. Quality assessment and data extraction
The methodological quality of the included studies was independently assessed by two authors (J.H.P. and S.P.) using the Cochrane Risk of Bias Tool for RCTs [29,31], and the second author (J.O.) would be consulted when disagreement appeared. The items on the tool were divided into seven specific domains: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); and (7) other sources of bias. The assessment in each domain was achieved by assigning a judgement from among the three options available for that domain (e.g., ‘ Low risk of bias’, ‘ High risk of bias’, and ‘ Unclear risk of bias’). Full details are given in Figs. 1, 2.
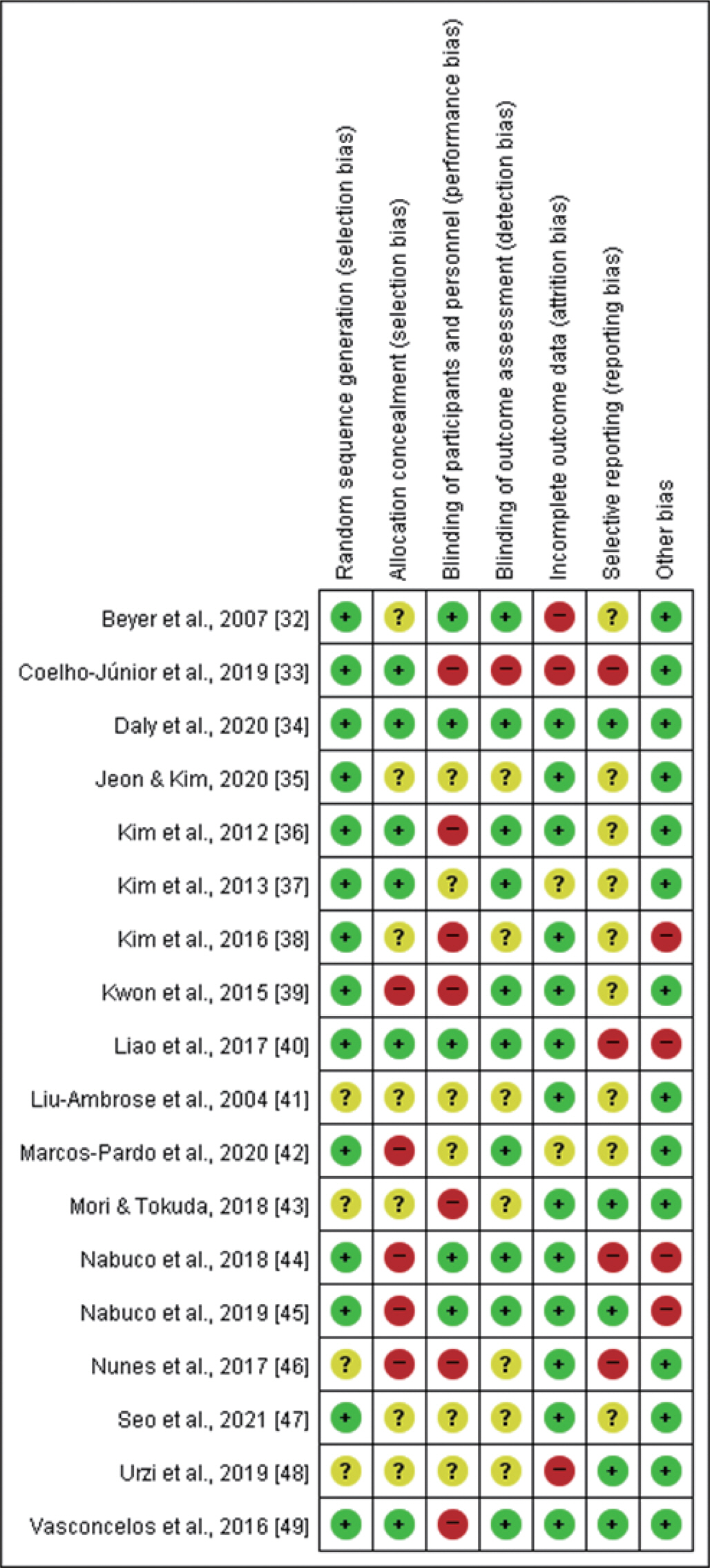
Risk of bias summary: judgements about each bias item for each study. (+) indicates a low risk of bias, (?) indicates an unclear risk of bias, and (−) indicates a high risk of bias.
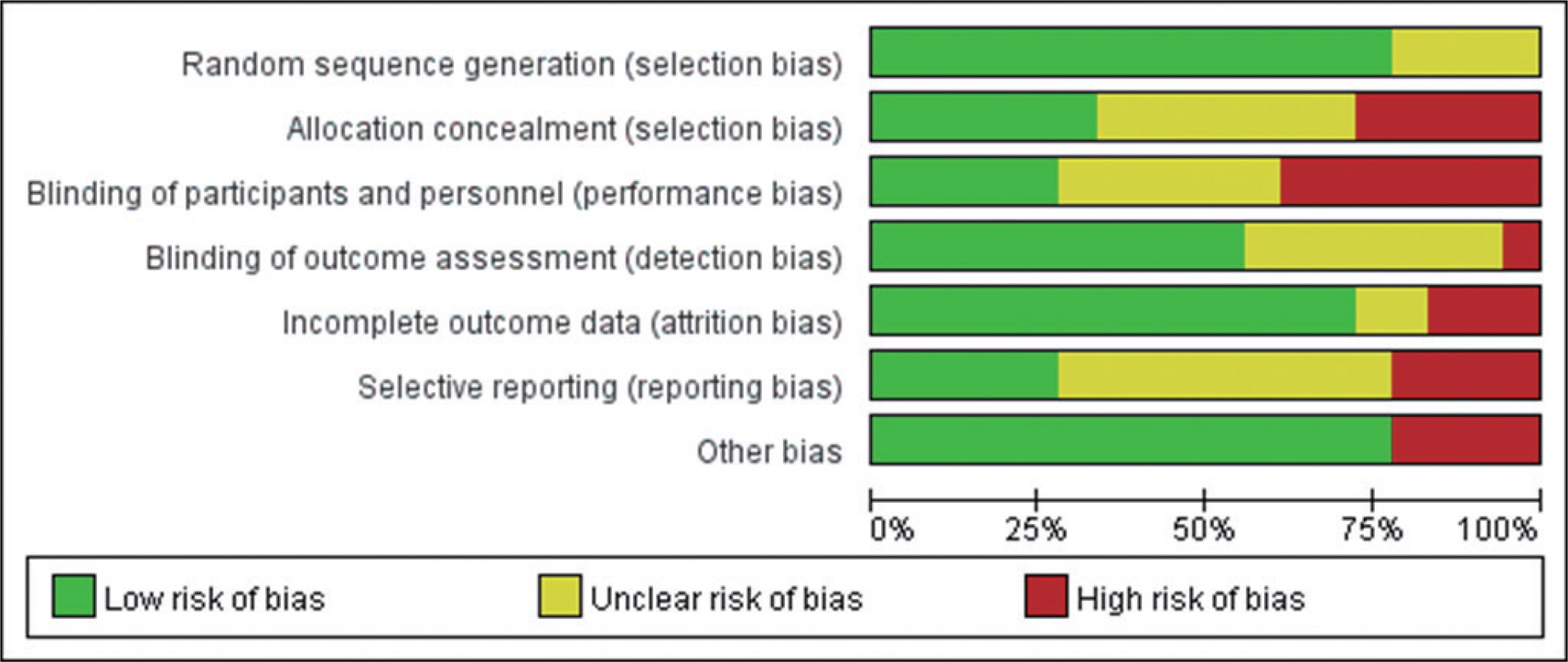
Risk of bias graph: review authors’ judgements about each risk of bias item, presented as percentages across all included studies.
Studies included in our meta-analysis were assessed as having a high or unclear risk of random sequence generation (4/18), allocation concealment (12/18), blinding of participants and personnel (13/18), blinding of outcome assessment (8/18), incomplete outcome data (5/18), selective reporting (13/18), and other sources of bias (4/18). Supplementary Table S1 (available at https://doi.org/10.15857/ksep.2022.00024) is included to explain the criteria for the judgement for each entry in the Cochrane risk of bias tool for RCTs [29,31].
5. Statistical analysis
Descriptive data of the participants’ characteristics are presented as means±standard deviations. The meta-analytic statistics were conducted using Stata/MP 16.0 (Stata Corp., College Station, TX, USA). The standardized mean difference (SMD), the number of subjects, and the standard error of the SMD for each study were used to quantify changes in the dependent variable when comparing the intervention (RT and/or PS) with the control groups (non-training control conditions or same conditions with a placebo). SMDs for each study were calculated using Hedges's g. SMDs were weighted by the inverse of variance to calculate an overall effect and its 95% confidence intervals (CIs). Considering the participants’ characteristics (postmenopausal women), types of interventions (RT and/or PS), and existence of methodological heterogeneity across the included studies, the random effect model was used to quantify pooled SMD of the included studies. We categorized the magnitude of SMD (MSMD) in accordance with Cohen's criteria [50]: small, SMD=0.2; medium, SMD=0.5; and large, SMD=0.8.
Subgroup analysis for the effect of RT on UGS was performed to further verify the differences in types of RT interventions. To perform this analysis, we classified studies based on the types of RT: ‘ RT-only intervention’ [33,35,38–40,46–48], ‘ RT combined with power training (PT)’ [33,49], and ‘ RT combined with BT’ [32,36,37,41,42]. Additionally, subgroup analysis for the additive effect of RT and PS on UGS was carried out to investigate the differences in types of PS interventions. For this analysis, we classified articles based on the types of PS: ‘ Whey protein (WP)’ [43–45], ‘ Multinutrient-fortified milk drink (MFMD)’ [34], and ‘ Leucine-enriched EAA’ [36,38].
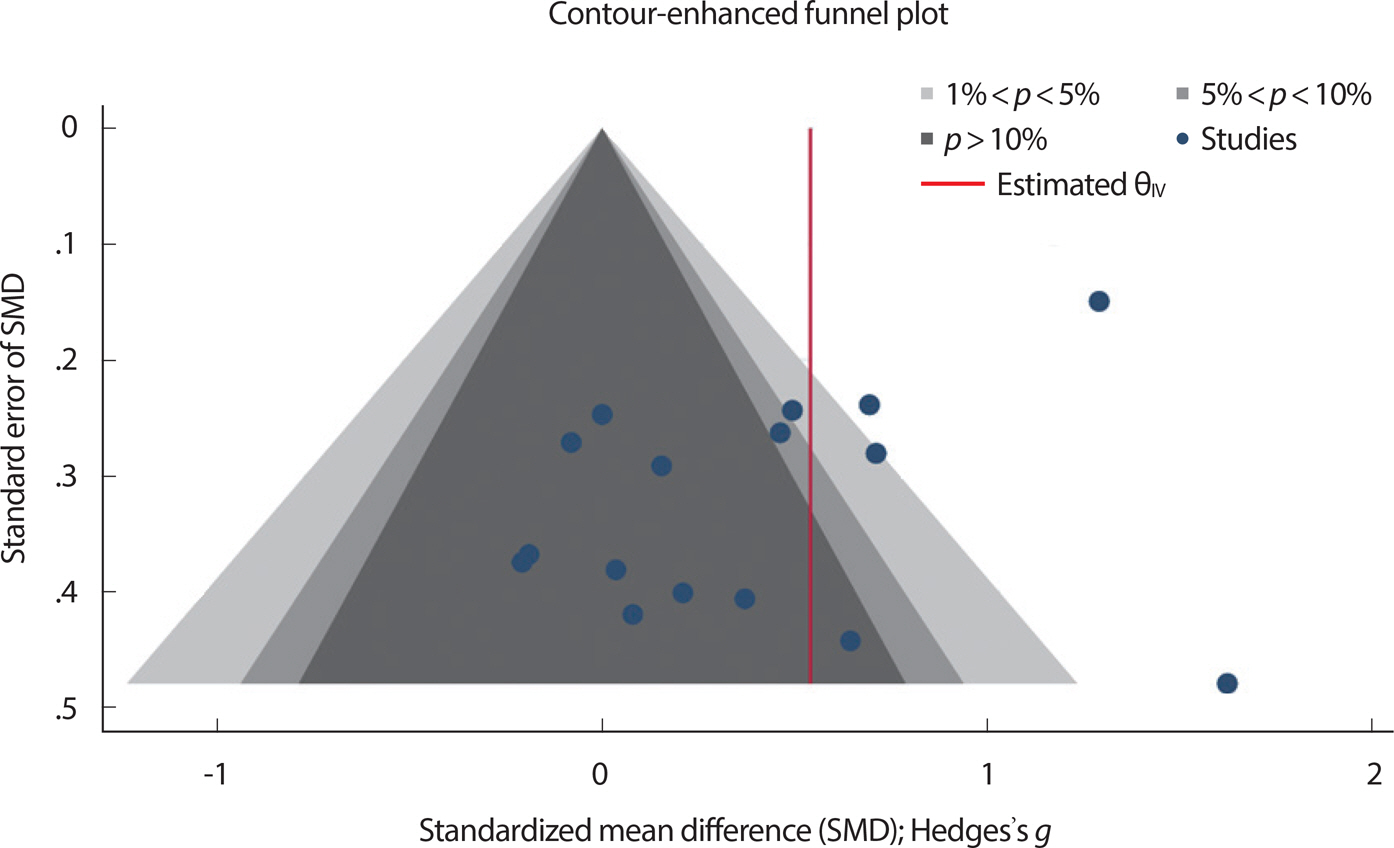
Statistical heterogeneity was assessed using Cochran's Q statistic and I2 statistic. All the performed meta-analyses evidenced the presence of substantial heterogeneity, defined as a Q statistic p value below 0.10 and the I2 ≥50% [31]. The random effect model was adopted for this meta-analysis due to the heterogeneity between studies. Furthermore, the possibility of publication bias was estimated with a visual inspection of the funnel plot using a contour enhanced funnel plot when there were at least 10 studies included in the meta-analysis [31]. If funnel plot asymmetry was detected, the trim-and-fill method was used to provide an estimate of the number of missing studies, and derive an adjusted intervention effect by performing a meta-analysis including the filled studies [29,31].
RESULTS
1. Literature search results
Fig. 3 demonstrates the study selection flow chart. The systematic literature search yielded 608 potentially eligible articles. From these 608 articles, 153 duplicate articles were excluded. After reviewing the title and abstracts, 421 irrelevant articles were excluded. The remaining 34 publications were downloaded for further review of the full text. Eighteen of those were excluded due to the following reasons: two studies were not available in English; nine studies lacked available UGS data; three studies did not include RT intervention; and four studies did not include a non-training control group. Additionally, from the search of reference lists of the included studies, we added two more articles. Therefore, the current systematic review and meta-analysis included 18 articles.
2. Study characteristics
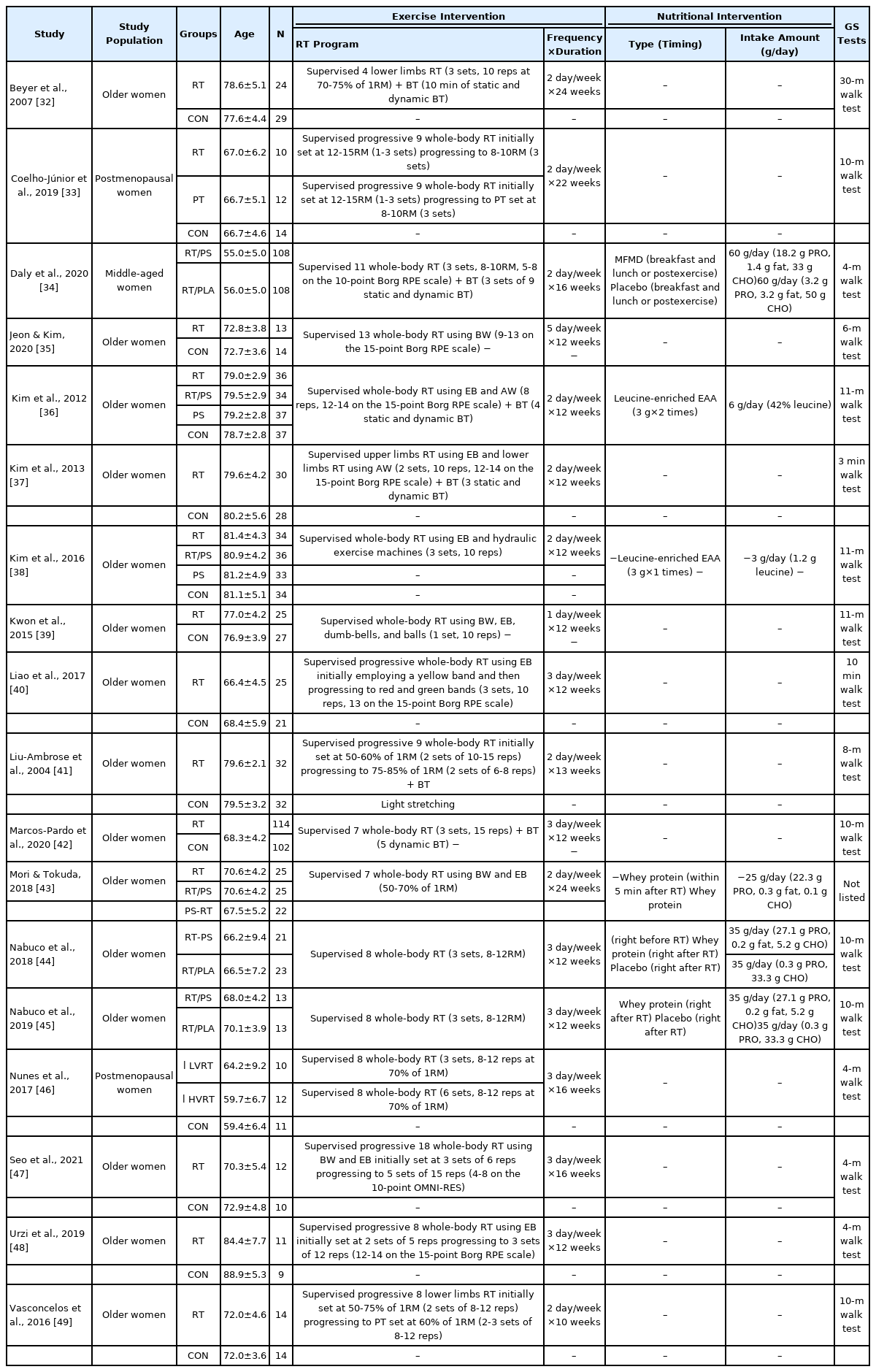
Table 1 displays the characteristics of the 18 studies [32–49] included in the systematic review and meta-analysis. In total, 1,294 women par-ticipated in the interventions, with a mean age of 70.8±8.9 years. Four-teen studies [32,33,35–42,46–49] investigated the effect of RT on UGS. Two studies [36,38] verified the effect of PS on UGS. Furthermore, six studies [34,36,38,43–45] analyzed the additive effect of RT and PS on UGS by comparing the effect between RT combined with PS and RT-only intervention.
3. The effect of RT on UGS
Fig. 4 presents the meta-analysis results of the effect of RT on UGS in postmenopausal women. The pooled SMD calculated using the random effect model was 0.40 (95% CI, 0.12-0.68; p =.006; MSMD, small), indicating a significant increase in UGS after RT. The I2 statistic indicated statistical heterogeneity (I2, 71.7%; p <.10). According to the subgroup analysis, there was a significant increase in UGS after ‘ RT-only intervention (SMD, 0.30; 95% CI, 0.01 to 0.59; p =.046; MSMD, small; I2, 34.6%)’. ‘ RT combined with BT’ also demonstrated a significant effect on UGS (SMD, 0.65; 95% CI, 0.17-1.13; p =.008; MSMD, medium), and the I2 statistic indicated statistical heterogeneity (I2, 82.5%; p <.10). In contrast, there was no significant increase in UGS after ‘ RT combined with PT (SMD, −0.08; 95% CI, −0.62 to 0.45; p =.765; MSMD, trivial; I2, 0%)’.
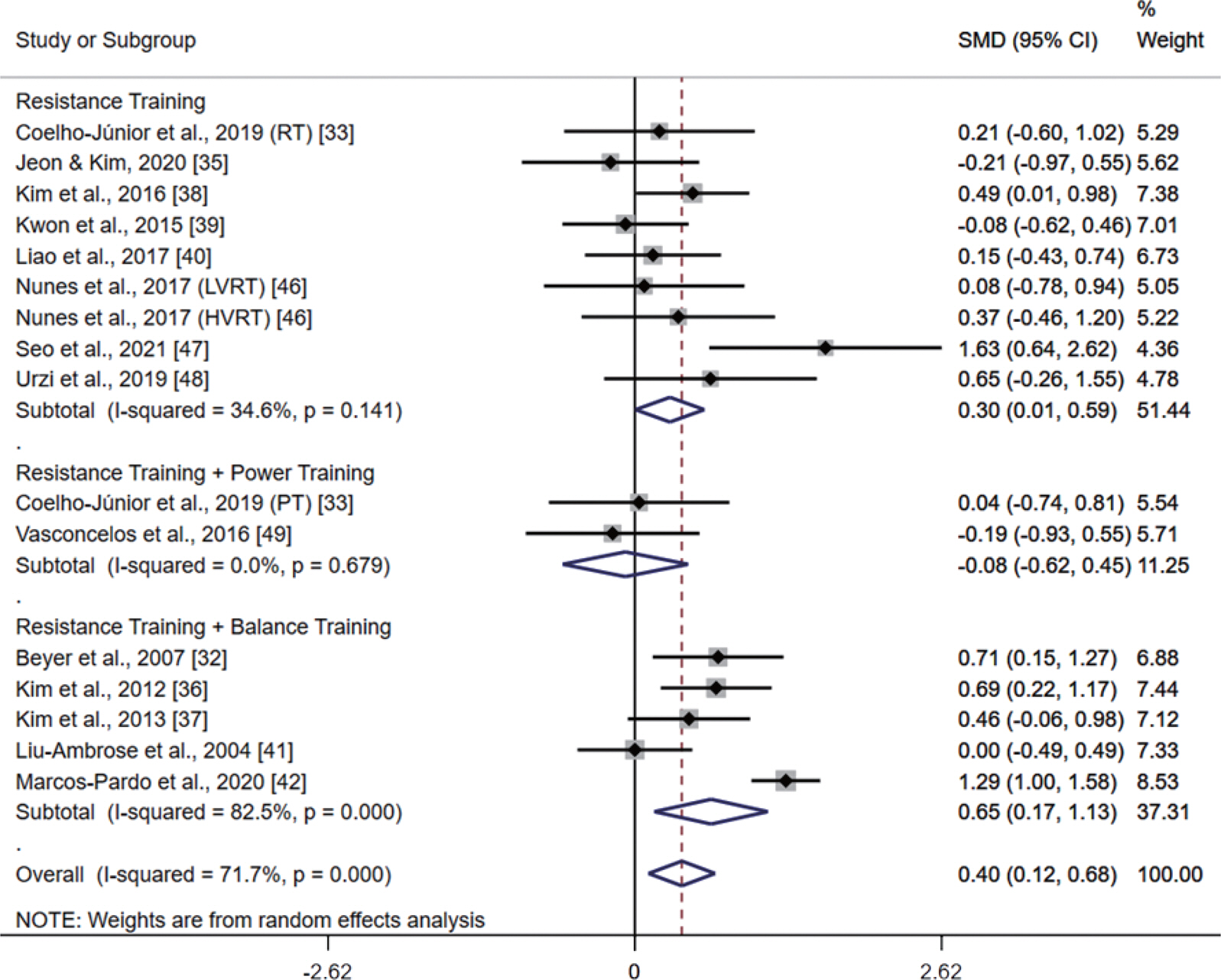
Meta-analysis results of the effect of resistance training (RT) on usual gait speed (UGS) in postmenopausal women. Forest plot demonstrates standardized mean differences (SMD) with 95% confidence intervals (CIs) for sixteen studies or subgroups. Subgroup analysis shows the results for the difference among RT, RT combined with power training, and RT combined with balance training. The diamond at the bottom shows the pooled SMD with the 95% CI for all studies following a random effect meta-analysis, and the size of the plotted squares indicates the relative statistical weight of each study. LVRT, low-volume resistance training; HVRT, high-volume resistance training; PT, power training.
4. The effect of PS on UGS
Fig. 5 presents the meta-analysis results of the effect of PS on UGS in postmenopausal women. The pooled SMD calculated using the random effect model was −0.17 (95% CI, −0.80 to 0.46; p =.601; MSMD, trivial), indicating a non-significant difference between PS groups and control groups in UGS. The I2 statistic indicated statistical heterogeneity (I2, 71.8%; p <.10).
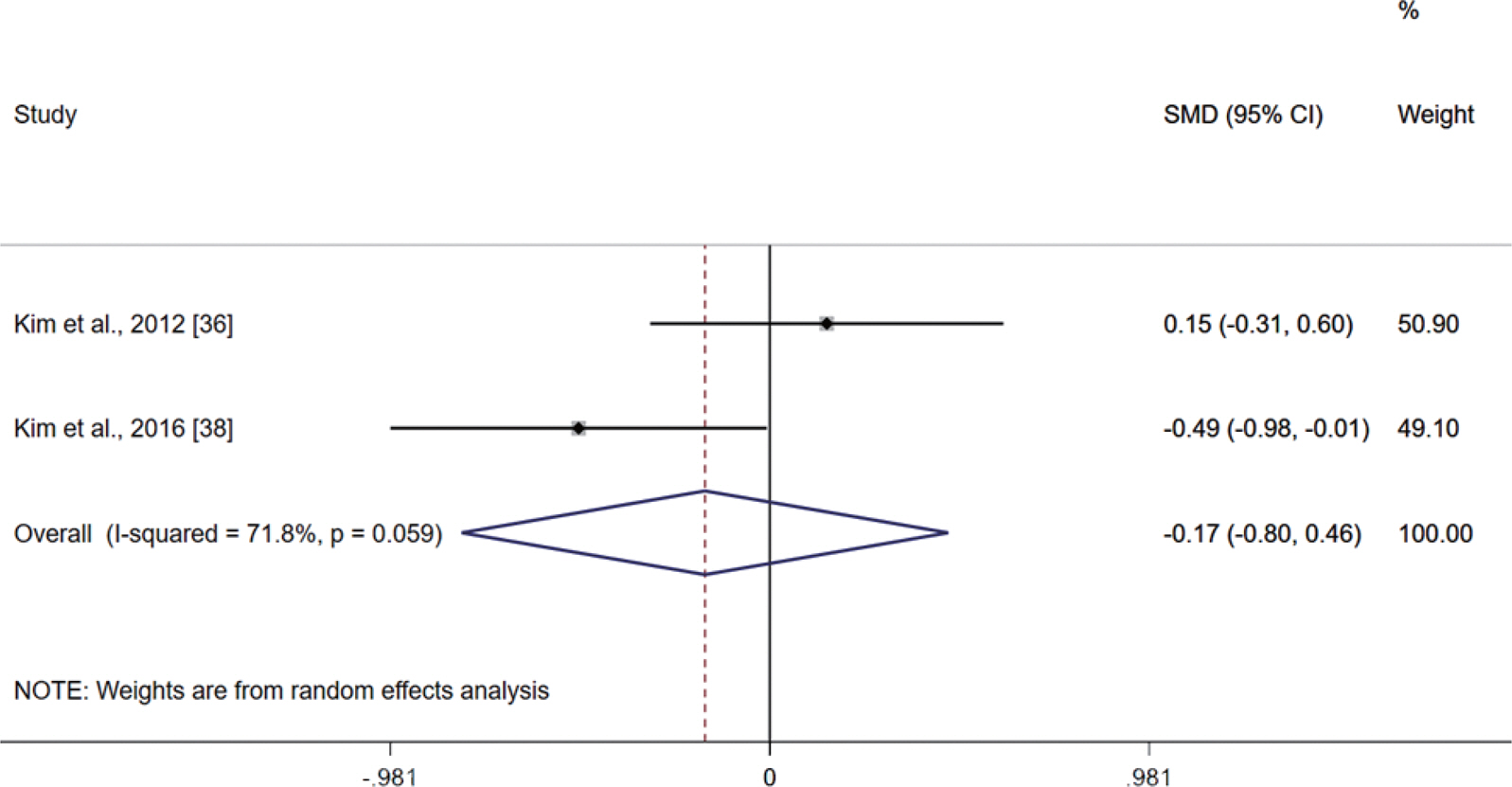
Meta-analysis results of the effect of protein supplementation on usual gait speed in postmenopausal women. Forest plot demonstrates standardized mean differences (SMD) with 95% confidence intervals (CIs) for two studies. The diamond at the bottom shows the pooled SMD with the 95% CI for all studies following a random effect meta-analysis, and the size of the plotted squares indicates the relative statistical weight of each study.
5. The additive effect of RT and PS on UGS
Fig. 6 presents the meta-analysis results of the additive effect of RT and PS on UGS; these results were determined by comparing the effect between RT combined with PS and RT-only intervention in postmenopausal women. The pooled SMD calculated using the random effect model was −0.06 (95% CI, −0.36 to 0.24; p =.699; MSMD, trivial), indicating a non-significant difference between RT combined with PS groups and RT-only intervention groups on UGS. The I2 statistic indicated statistical heterogeneity (I2, 60.5%; p <.10). The subgroup analysis did not find any additive effect on UGS after ‘ RT combined with WP supplementation (SMD, 0.25; 95% CI, −0.07 to 0.58; p =.128; MSMD, small; I2, 9.7%)’, ‘ RT combined with MFMD supplementation (SMD, −0.40; 95% CI, −0.67 to −0.13; p =.004; MSMD, small)’, and ‘ RT combined with leu-cine-enriched EAA supplementation (SMD, −0.30; 95% CI, −0.67 to 0.07; p =.108; MSMD, small; I2, 18.4%)’.
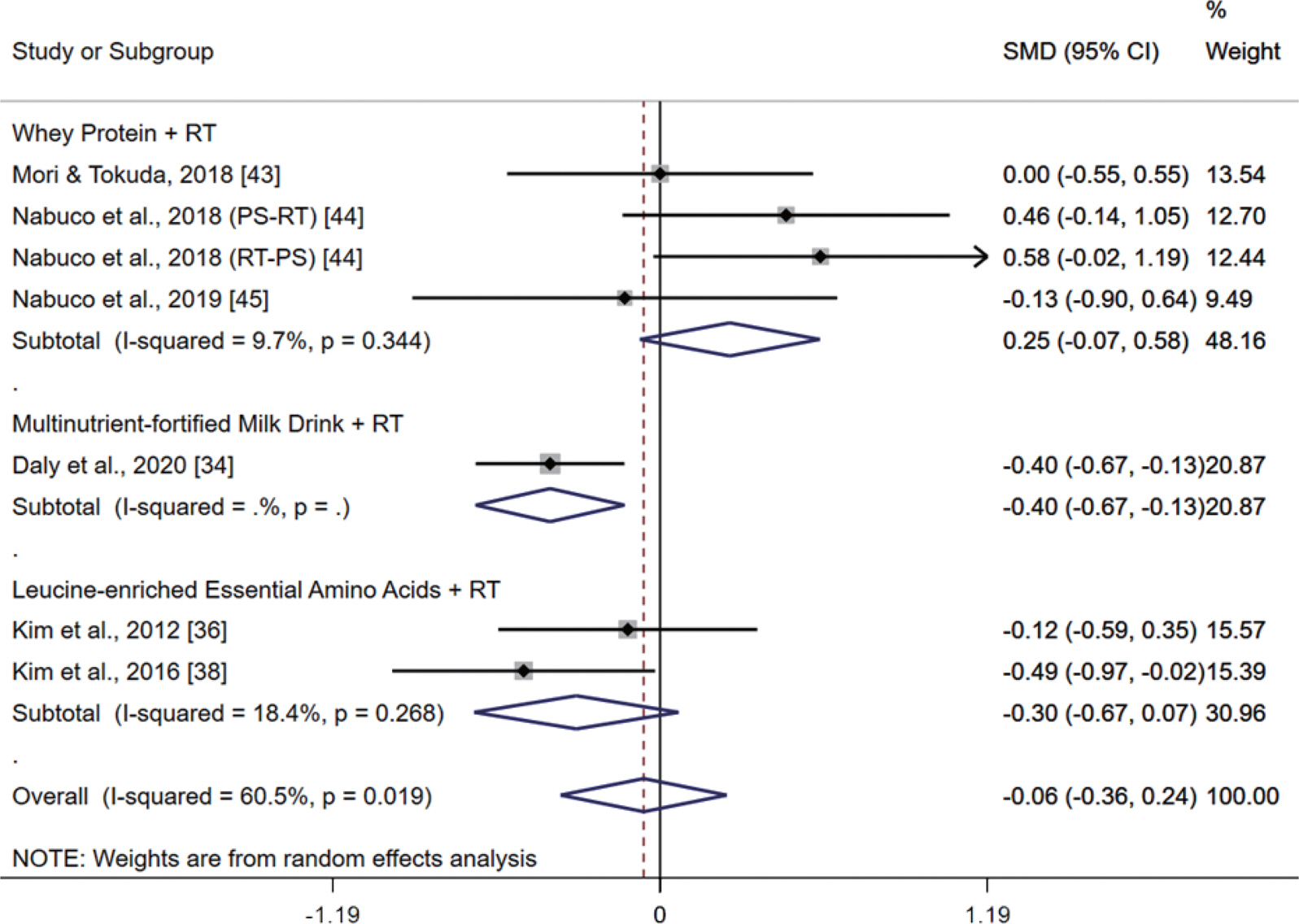
Meta-analysis results of the additive effect of resistance training (RT) and protein supplementation (PS) on usual gait speed by comparing the effect between RT combined with PS and RT-only intervention in postmenopausal women. Forest plot demonstrates standardized mean differences (SMD) with 95% confidence intervals (CIs) for seven studies or subgroups. Subgroup analysis shows the results for the difference among types of protein supplements. The diamond at the bottom shows the pooled SMD with the 95% CI for all studies following a random effect meta-analysis, and the size of the plotted squares indicates the relative statistical weight of each study.
6. Publication bias
In the present review, we were able to estimate the publication bias of only one meta-analysis, which investigated the effect of RT on UGS, and included 14 studies [32,33,35–42,46–49]. Fig. 7 presents the contour-en-hanced funnel plot depicting the effect of RT on UGS. The funnel plot showed slight asymmetry, suggesting the possible presence of missing studies on the bottom right hand side based on the pooled SMD (red line). However, the plausibility of publication bias being the underlying cause of this asymmetry is reduced as most of this area includes regions of statistical significance indicated by lighter shading [31]. Additionally, to further verify this asymmetry arising from publication bias, we uti-lized the trim-and-fill method, which indicated that the effect of RT on UGS remained significant after imputing three possible missing articles (adjusted SMD, 0.529; 95% CI, 0.28-0.78). These findings indicate that this asymmetry does not pose a challenge to the plausibility of the results of our meta-analysis.
DISCUSSION
To the best of our knowledge, this is the first study that included only RCTs to verify the meta-effects of RT and/or PS on improvement in UGS specific to postmenopausal women. The key findings of this systematic review and meta-analysis were that RT-only intervention significantly improved UGS in postmenopausal women, and RT combined with BT further improved UGS. In addition, there was no significant improvement in UGS after PS intervention, and no additive effect on UGS after RT combined with PS.
The decline in estrogen production during the menopausal transition has a harmful effect on bone mass density, muscle mass, muscular strength, muscular power, and even physical function [11–13]. Regular exercise, especially RT, has been recommended as an efficient strategy to improve muscle mass, muscular strength, and physical function, including UGS [16,17]. According to the subgroup analysis, there was a significant positive meta-effect on UGS after RT-only intervention. This finding is consistent with a previous meta-analytic study that found a significant meta-effect of RT with high intensity at 70-80% of one repetition maximum (1RM) on UGS in older adults [18]. In contrast, in our meta-analysis, all but one RT-only intervention studies applied moderate intensity RT [35,38–40,46–49]; the remaining one study applied a high intensity RT [33]. UGS is a standardized tool for evaluating physical performance and function in gerontological research, as it is positively associated with muscular strength of lower-limb and trunk muscles [6,8]. Although RT with high intensity (90% of 1RM) has been seen to demon-strate greater strength gains than RT with moderate intensity (70% of 1RM) in older adults [51], RT with moderate intensity may also have a beneficial effect on UGS in our study. Thus, considering the previous meta-analysis and our results, both moderate and high-intensity RT can be expected to be an effective method for improving UGS as well as muscular strength in older adults including postmenopausal women. Furthermore, even if there was no significant improvement in UGS after RT combined with PT in our study. However, the number of studies included in this analysis was too low to draw conclusion, so further RCTs are needed to determine the effects of various types of RT on UGS in postmenopausal women.
Functional balance and gait disorders are major risk factors for future disability and fall risk in older adults [52]. Moreover, age-related decrease in functional balance ability has been significantly associated with slower UGS in older adults aged 50-85 years [53]. For this reason, diminished UGS in aging has been strongly associated with mobility disability and fall risk [2,54]. Thus, it seems necessary to establish strategies to prevent functional balance impairment and disrupted gait (e.g., slower UGS). In this regard, several meta-analyses have verified the effects of a combined RT program (RT combined with BT) on functional balance impairment and UGS. Abbema et al. [18] reported a significant effect of an RT-only program with high intensity on UGS in older adults; they further found that the addition of BT did not result in any additional meta-effect of the RT program on UGS in older adults. On the contrary, Sherrington et al. [55] reported that RT combined with BT significantly reduced the rate of falls in older adults, whereas RT-only intervention had no significant beneficial effect. Considering the conflicting results of the two meta-analyses, it can be concluded that the effects of RT combined with BT on UGS have not been fully investigated. According to our subgroup analysis, there was a significant increase in UGS after RT combined with BT, and this favorable effect was larger than that of RT-only intervention.
Considering the abovementioned relationship between UGS and fall risk, this result is likely to support previous findings [55]. Moreover, in contrast to Abbema et al. [18], who applied high intensity RT in their study, moderate intensity RT was applied in all of RT combined with BT studies in our meta-analysis [32,36,37,42], except for one study that applied a high intensity RT [41]. These findings collectively suggest that al-though moderate intensity RT is enough to improve UGS and decrease fall risk, adding BT to RT programs further improve UGS in postmenopausal women. Importantly, as static postural control, dynamic balance, and motor control play important roles in regulating UGS, BT has been recommended for preventing balance impairment and improving gait function in older adults [53,56].
PS has been suggested as an effective way to prevent age-related frailty, but protein intake tends to decrease with aging [24]. Thus, additional PS seems to be necessary to prevent a decline in physical functions, including UGS, of older adults. EAA, especially leucine, have a key role in regulating MPS [57]; hence, it has been reported that a high proportion of leucine (41%) in EAA can reverse a reduced MPS in older adults [58]. In addition, a recent study concluded that 1.5 g of leucine-enriched EAA (0.6 g leucine) was enough to stimulate MPS maximally in older women compared to 6 g of leucine-enriched EAA (2.4 g leucine) or even 40 g of WP [59]. As illustrated in Fig. 5, however, there was no significant meta-effect of PS (3-6 g of leucine-enriched EAA; 1.2-2.5 g leucine) on UGS in postmenopausal women. This result supports previous findings, which reported no significant increases in lower-limb muscular strength after 12 weeks of leucine intake (7.5 g/day) in older men [60] or 7.5 g of EAA intake (1.4 g leucine) in older women [61]. A previous meta-analysis reported no significant meta-effects of PS on walking speed and lower-limb muscular strength in older adults [62]. In this regard, according to a three-year follow-up study, higher protein intake (≥1.1 g/kg/day) was associated with a lower likelihood of frailty, including slower UGS, when compared to lower protein intake in older women (<1.1 g/kg/day) [23]. Furthermore, a study employing a 12-week intervention program for older adults reported that the group whose protein intake was 1.5 g/kg/ day had significantly higher muscle mass and UGS compared with the group that had a protein intake of 0.8 g/kg/day; no significant differences were found between the groups with protein intakes of 1.2 g/kg/day and 0.8 g/kg/day [26]. Thus, prior to verifying the effect of PS on UGS, it is important to consider the habitual protein intake of the participants. However, the habitual protein intake was not reported in the studies included in our meta-analysis [36,38]. Based on previous research, an increase in the protein intake of about 0.55 g/kg/day is sufficient to improve muscle mass in older men, but not in older women, because postmenopausal women have greater anabolic resistance of MPS than older men [63,64]. Taken together, these studies suggest that the dose of PS (3-6 g of leucine-enriched EAA) included in our meta-analysis may have been insufficient to meet the criteria (1.5 g /kg/day) to improve physical functions, including UGS. Therefore, further studies are needed to determine the effects of various doses and types of PS on UGS in postmenopausal women.
PS alone without other interventions (e.g., RT) may be insufficient to increase muscular fitness and physical function including UGS. Importantly, muscle protein breakdown, which is increased after RT, is negated by an optimal PS, and this maximizes postexercise MPS and enhances further adaption to RT, as indicated by muscle hypertrophy and increases in muscular strength and physical performance [65]. Thus, we additionally investigated whether there was an additive effect of RT and PS on UGS by comparing the effect between RT combined with PS and RT-only intervention. As illustrated in Fig. 6, in contrast to our hypothesis, there was no additive effect on UGS after RT combined with PS was compared with RT-only intervention. This result is in line with two previous meta-analyses that reported no additive effect on muscular fitness and physical function, including UGS, after RT combined with PS was compared with RT-only intervention in older adults [62,66]. A meta-an-alytic and meta-regression study found that the impact of PS on gains in muscle mass during prolonged RT declined with increasing age [67]. This is because of an anabolic resistance defined as the reduced ability of MPS in response to anabolic stimuli such as PS (e.g., protein and amino acids) and RT in older adults [64,68]. Therefore, a larger amount of protein supplements may be required for optimal MPS after RT in older postmenopausal women than in young women. It has been reported that post-RT rates of MPS are saturated with 20 g of protein in young adults, whereas older adults optimally respond to higher protein doses (40 g) after RT [69]. However, the amount of protein supplements included in the studies that formed our meta-analysis was smaller than this recommended protein dose of 40 g; for instance, dosage for WP ranged from 22.3-27.1 g [43–45], dosage for MFMD was 18.2 g [34], and dosage for leucine-enriched EAA ranged from 3-6 g of EAA [36,38]. Among them, a small but non-significant effect was observed in the RT combined with WP supplementation (SMD, 0.25; 95% CI, −0.07 to 0.58) with the highest protein content. Thus, these protein doses may have been insufficient to produce additive effects of RT combined with PS on physical functions, including UGS. Therefore, further studies are needed to determine the quantity of optimal protein doses that would produce additive or synergistic effects of RT combined with PS on physical functions specific to postmenopausal women.
This systematic review and meta-analysis had some limitations. First, our review was confined to articles published in English, which may have reduced the number of included articles. Second, the slightly asym-metrical funnel plot suggested the possible presence of missing articles and the possibility of an underestimated SMD, although the meta-effects of RT on UGS remained significant after imputing three possible missing studies through the trim-and-fill method. Third, it was impossible to perform the subgroup analysis of the effect of various types, doses, and timings of PS on UGS in postmenopausal women, because only a few studies on this issue were available. Last, we were unable to perform an age-specific subgroup analysis for the effect of RT and/or PS on UGS, because the number of studies was too small to divide by the subgroup. Thus, it is necessary to conduct further meta-analyses to verify the effects of RT and/or PS on UGS specific to postmenopausal women.
CONCLUSION
The present review suggested that RT demonstrated a significant ef-fect on UGS in postmenopausal women, and adding BT to RT programs further improved UGS. In addition, there was no significant improvement in UGS after PS in postmenopausal women, and no additive effect on UGS after RT combined with PS. However, considering the limitations of the present review, further systematic reviews and meta-analyses including a more diverse range of studies are required to accurately verify the meta-effects of RT and/or PS on physical functions, including UGS, in postmenopausal women.
Supplementary Material
Supplementary Table S1.
Judgement and a support for the judgement for each entry in the Cochrane risk of bias tool
Notes
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Conceptualization: JH Park, S Park; Data curation: JH Park, J Oh; Formal analysis: JH Park, J Oh; Methodology: JH Park, S Park; Visual-ization: JH Park, J Oh; Writing-original draft: JH Park; Writing-review & editing: JH Park, S Park.