The Effects of External Vibration on Coordination Strategies of Multi-Muscles during Voluntary Isometric Torque Production
Abstract
PURPOSE
To investigate the effect of muscle vibration on performance accuracy and multi-muscle coordination pattern during voluntary isometric knee extension torque production.
METHODS
The subjects were tested under two conditions of external vibration frequencies (90 Hz vibration (VIB)&no-vibration (NVIB)) with three levels of torque magnitudes of 20% (MVT20), 40% (MVT40), and 60% of maximal voluntary torque (MVT60). The subjects were instructed to perform a submaximal isometric ramp task and matched the produced torque with the torque template shown in the screen as accurately as possible. External vibration was applied to the rectus femoris (RF).
RESULTS
The performance error (RMSENORM) was reduced in 60% of MVT (MVT60) in both ramp and SS phases, and the iEMGAGO was significantly reduced by vibration under the same torque conditions in the SS phase. In addition, the muscle-mode (M-mode) composition was found to be different in the VIB and NVIB in the SS phase. We found that the VIB condition showed co-contraction M-modes and mixed M-modes. However, there was no significant difference in the ramp phase under all conditions.
CONCLUSIONS
The neurophysiological changes due to muscle vibration may positively affect the task characteristics and steps that require accurate torque generation and provide information for the quantitative understanding of multi-muscle coordination of vibration.
Keywords: Muscle vibration, Knee extension torque, Accuracy, Multi-muscle coordination
INTRODUCTION
External vibratory stimulation is of diverse effects on the peripheral elements within the motor and sensory structure in humans [ 1– 3]. In particular, the external vibration on muscles or tendons causes the instantaneous and phasic changes in the length of muscle fibers, resulting in the excitation of the sensory endings of afferent fibers [ 4– 6]. The excitation of afferent nerves induced by the vibratory stimulation causes reflexive muscle contraction known as tonic vibration reflex (TVR) [ 7, 8], and the effect of muscle contraction associated with the TVR extends to the muscle activation for opposing action. Further, the mechanical and physiological responses as to the muscle activation to the vibratory stimulation depend on the properties of the external vibration, such as the frequency [ 9], amplitude [ 10, 11], and duration [ 12, 13]. In other words, short-term vibratory stimulation was of positive effect on the muscular strength for the maximal force production capability [ 1, 2, 14, 15], while prolonged stimulation decreased muscular strength accompanied by a reduced magnitude of H-reflex along with increased threshold level for the action potential [ 13]. A recent study revealed that the increased torque magnitude with vibration was associated with increased agonist activities and decreased antagonist activities resulting in reduced muscle cocontraction among the agonist and antagonist muscles during maximal voluntary contraction [ 14]. These results imply that short-term external vibration could modulate the organization of multi-muscle activities. Indeed, a set of muscles is simultaneously involved during a simple motor task, even in a simple single joint flexion and extension, whole-body swaying, etc. However, there remains many unanswered questions about how the vibratory stimulation on the specific muscle affects the covariation and organization of the multiple muscles during the submaximal force production. In general, we would like to emphasize that the investigation on a proper organization of the muscles against external stimulus would be a prerequisite for the understanding of accurate or inaccurate motor performance. Notably, the involved muscles are coordinated in such a way that the muscles are united into a few groups (e.g., agonist and antagonist groups). In other words, the control strategies to govern multi-muscles may not send command signals, which define individual parameters of muscle activities, to individual muscles but specify a few parameters that affect groups of muscles. The notion of muscle mode (M-mode) has been introduced. The organization of M-modes is beneficial to some extent in reducing the number of variables governed by the controller. As such, M-mode is an activated muscle group that is individually manipulated by the central nervous system (CNS) and reduces the problem of motor redundancy [ 15– 18]. This study employed a submaximal isometric ramp task using the knee joint in isometric conditions. The knee joint torque and the elec-tromyography (EMG) of a set of lower extremity muscles (e.g., agonist and antagonist muscles) were measured. The precise production of knee extension torque is accompanied by proper coordination of the involved muscles. This study investigates the effect of muscle vibration on the accuracy of performance and muscle-modes (i.e., M-modes) during voluntary isometric knee extension torque production. Based on the knowledge of the aforementioned experimental results, we formulate the following two hypotheses. First, muscle vibration will decrease performance errors in tasks that require accuracy at the submaximal forces level by controlling the activity of agonist and antagonist muscles. Second, the M-modes will change with a muscle vibration in the submaximal force level task.
METHODS
1. Participants
Eight healthy male subjects (30±3.8 years, 174±2.3 cm, 77±9.32 kg, thigh fat thickness: 3.0±0.4 mm) participated in the study. The dominant leg was the right for all participants. The exclusion criteria for the subject recruitment included musculoskeletal injuries and dysfunction. The manual muscle testing of all the participants was under grade 5 [ 19], and the body mass indices were within a normal range [ 20]. The experimental procedure of the study was performed following the recommen-dations of the Seoul National University Institutional Review Board (IRB No. 1704/001-009).
2. Apparatus
Four vibrators (3 cm diameter, 0.7 cm height) were used to provide an external vibration on a specific muscle, and the properties of vibration stimulus (e.g., amplitudes, frequencies, and time) could be adjusted. A single force sensor (MC3A, AMTI, Watertown, MA USA) was used to measure the knee extension force. The force sensor was firmly fixed to the customized steel frame. The position of the sensor was adjusted between the knee and ankle joints according to the subjects’ length of the shank. The orientation of the force sensor was aligned in such a way that the z-axis force component was perpendicular to the long axis of the shank. The wireless sEMG (Trigno Wireless EMG System, Delsys, Natick, MA, USA) electrode was attached to the belly of the five lower limb muscles, including the vastus lateralis (VL), rectus femoris (RF), vastus medialis (VM), biceps femoris (BF), and semitendinosus (ST) ( Fig. 1B). All measurement systems were physically synchronized, and the force transducer and sEMG were all sampled at frequencies of 200 and 2,000 Hz.
Fig. 1.
Fig. 1.(A) Experimental set up of voluntary isometric torque production including submaximal isometric ramp tasks and MVT task. To perform the isometric knee extension torque, all subjects were fastened to a steel frame equipped with a force/torque sensor at 120 degrees of knee angles, respectively. The sensor was mounted with the z-axis perpendicular to the long axis of the shank. (B) Four vibrators were closely attached to the belly of the rectus femoris (RF) muscle. The surface EMGs were attached to the belly of the eight muscles without interference. (C) A sample of submaximal isometric ramp tasks data from the representative subject. A monitor providing real-time visual feedback of the knee extension torque was installed on the front of the subject. The template on the screen provided three magnitudes of target torque (20%, 40%, and 60% of MVT). Pre-activation of constant 5% MVT for the first 6-s and followed by a slanted line from 5% to one of the three torque magnitudes over the next 5-s (i.e., ramp phase), and another horizontal segment for the last 4-s (i.e., steady-state phase, SS). The vibration was given from 5-s to 15-s until the end of the trial for 10-s. 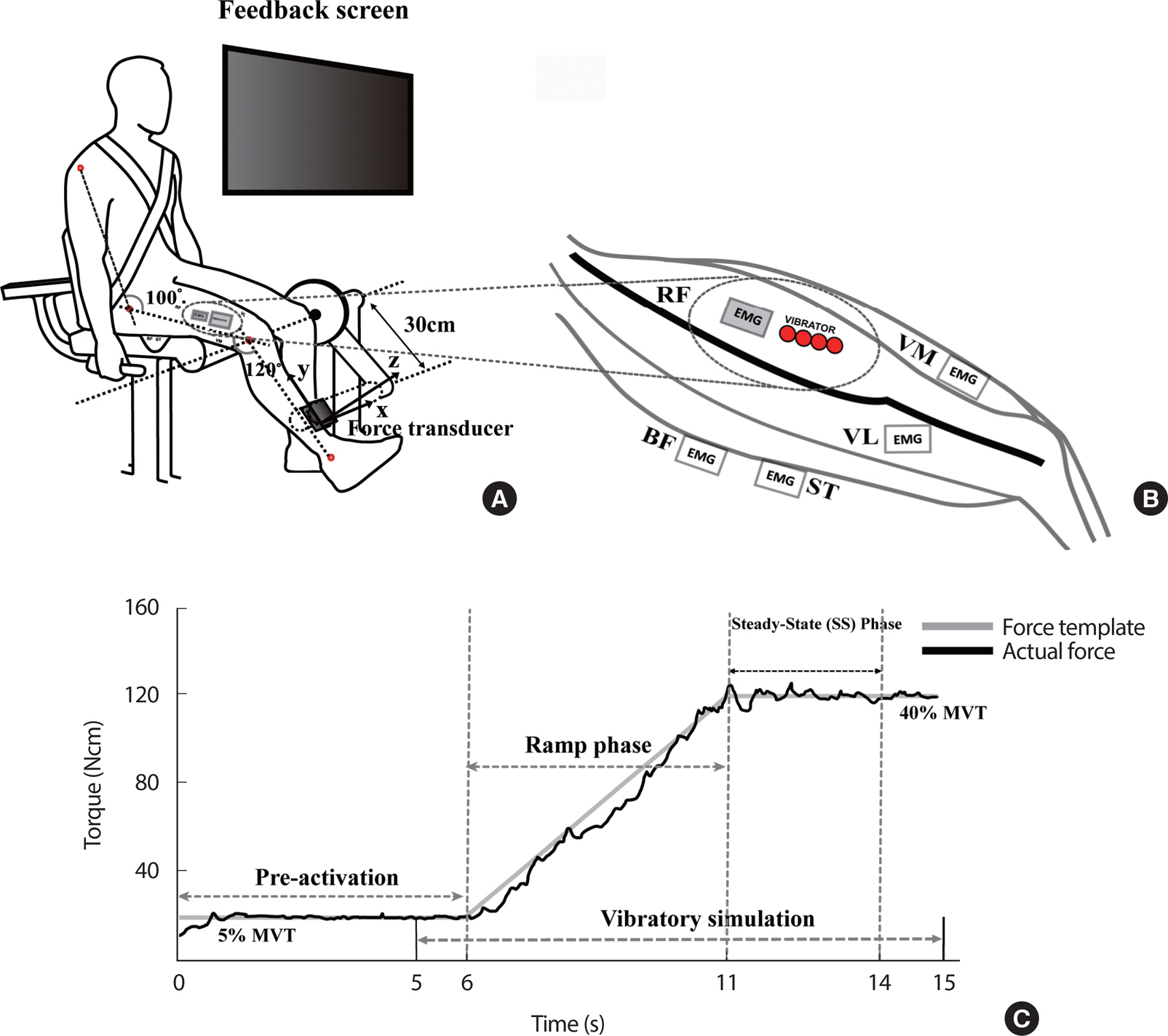
3. Experimental procedure
Each subject sat on the customized leg-extension machine, wore a fas-tening belt on his trunk, and held grips tightly with facing a screen for real-time force feedback ( Fig. 1A). The hip and knee joint angles with respect to the horizontal axis were fixed at 100° and 120° [ 16, 21]. The vibrators were attached along the periphery of the RF ( Fig. 1B), which is the most superficial muscle in the agonist muscles for the knee extension. For the vibration condition, the frequency and amplitude were set at 90 Hz and 10 mm, and the action of all vibrators was synchronized so that they were on and off simultaneously. The following tasks were tested under the vibration (VIB) and no-vibration (NVIB) conditions. The first task was the maximal voluntary torque (MVT) production task. The subject was instructed to maintain 100 Nm of isometric knee extension torque for the first 6-s as a pre-activation phase. After that, the subjects were required to perform the knee extension effort as hard as possible during the next 3-s. The second task was the submaximal isometric ramp tasks with three levels of torque magnitudes as terminal steady-state torque values. The conditions of the torque magnitudes included 20, 40, and 60% of MVC (i.e., MVT 20, MVT 40, and MVT 60). Subjects were instructed to produce the knee extension torque while matching the produced torque in real-time with the torque template shown on the computer screen as accurately as possible. In a single trial, the 15-s torque template consisted of a horizontal segment at 5% MVT for pre-activation of the involved muscles, followed by a slanted line from 5% to one of the three torque magnitudes over the next 5-s (i.e., ramp phase), and another horizontal segment for the last 4-s (i.e., SS phase) ( Fig. 1C). For the VIB condition, the vibration stimulus was provided 1-s prior to the initiation of the ramp phase and lasted until the end of the trial. Each subject performed twelve consecutive trials per condition. A 3-minute resting time was given between trials to wash out the vibration and fatigue effects.
4. Data Analysis
The force data were filtered using a zero-lag 4th-order low-pass Butterworth filter at 10 Hz cutoffs. The EMG data processing for data analysis was as follows: 1) application of notch filter to remove noise in the same frequency band (90 Hz) as the vibrator stimulus, 2) application of 10 to 450 Hz band-pass filter and second-order zero-lag Butterworth filter. All the current analysis variables were computed in the ramp and SS phases in a single trial and further averaged across multiple trials. Root-mean-squared error (RMSE) as an index of performance accuracy con-cerning the force/torque production was quantified for each trial. Further, the RMSE values were normalized by the values of template torque at corresponding conditions.
The integrated EMGs ( i EMGs) over for individual trials were computed. The i EMGs were formed into a matrix with 800 columns corresponding to five muscles (i.e., VL, RF, VM, BF, and ST) being analyzed and five rows corresponding to i EMG value stacked for the total of twelve trials. The cocontraction index (CCI) indicates the relative muscle activity of the antagonist muscles (i.e., i EMG ANT) to the overall muscle activities (i.e., i EMG TOT) calculated [ 22]. Since the required knee torque direction was extension, thereby, a group of antagonist muscles was BF and ST. Defining Muscle-Mode (M-modes) using principal component analysis (PCA), we extracted groups of muscles from the i EMG data. i EMG data were applied to group muscles depending upon the parallel scaling of the activation levels of the muscles during a submaximal isometric ramp task, and these groups of muscles were defined as M-modes (M 1-mode, M 2-mode, and M 3-mode). We are concerned with two experimental conditions with three levels of torque magnitudes (MVT 20, MVT 40, and MVT 60) i EMG data stacked into 9,600 by five matrices containing i EMG. For each subject, the obtained eigenvalues and principal components (PCs) were then considered. This allows the reduction of the 5-di-mensional muscle space into a smaller dimensional space (M-Mode space). The extraction of the number of M-modes based on the Kaiser criterion (eigenvalue >1). In the present study, the number of extracted modes was decided to be three [ 23, 24]. Sets of three modes accounted for a percentage of variance in line with previous studies [ 25– 27]. To investigate the composition of M-modes between VIB and NVIB conditions, we analyzed the different types of M-modes. They were cocontraction M-modes (coactivation of agonist and antagonist), reciprocal M-modes (consisted of only agonist or antagonist), and mixed M-modes. The mixed M-modes could involve at least one more muscle loaded significantly on the agonist or antagonist.
5. Statistical Analysis
The statistical analysis was performed using SPSS 24.0 (IBM, USA). Descriptive statistics were used the data are presented in the text as means±standard errors (SE). Two-way repeated ANOVA was used with factors including Vibration (2 levels: VIB and NVIB) and Torque (3 levels: MVT20, MVT40, and MVT60).
A paired t-test was used to confirm the difference of the one-to-one correspondence between VIB and NVIB in each %MVT condition. In order to test the comparison of the percent variance explained by M-modes between the VIB effect, two-way repeated ANOVA was used with z-transformed using Fisher’ s z-transformation. Significant loadings coefficients for each M-mode were selected to be greater than <0.4. For all statistical tests, a p-value less than <.05 was set as a measure of signif-icance.
RESULTS
1. Root Mean Square Error (RMSE NORM)
All the following variables were analyzed in the two phases, including the ramp and SS phases. First, the RMSE NORM as a performance accuracy index was computed. During the ramp phase, the RMSE NORM was smaller in the VIB condition than in the NVIB condition, with there being no significant effect on torque ( Fig. 2A). These results were supported by a two-way repeated measure ANOVA on the RMSE NORM with factor Vibration (2 levels: VIB and NVIB) and Torque (3 levels: MVT 20, MVT 40, and MVT 60), which showed a significant main effect of Vibration ( F[ 1, 7] = 14.082, p <.001, ηp²=0.668) with Vibration × Torque factor interaction ( F[ 2, 14] =11.129, p <.001, ηp²=0.614). In particular, the VIB effect (i.e., decreased RMSE NORM with the vibration) was significant at MVT 60 conditions confirmed by posthoc pairwise comparisons ( p <.001). During the SS phase, furthermore, the RMSE NORM was smaller in the VIB condition than in the NVIB condition ( F[ 1, 7] =9.941, p <.001, ηp²=0.587), while no significant effect of Torque ( Fig. 2B). In particular, the vibration effect (i.e., decreased RMSE NORM with the vibration) was significant at MVT 60 conditions only, which was confirmed by posthoc pairwise comparisons ( p <.05).
Fig. 2.
Fig. 2.All the variables are presented mean±standard error bar of RMSE NORM depending on all the torque conditions and the VIB & NVIB in (A) ramp & (B) SS phase. 
2. Integrated EMG (iEMG NORM)&Cocontraction Index (CCI)
Secondly, the i EMGs of the groups of agonists ( i EMG AGO) and antagonist muscles ( i EMG ANT) were computed. Generally, i EMG AGO and i EMG ANT increased significantly with the magnitudes of torque ( p <.01). Nevertheless, during the ramp phase, i EMG AGO no significant effect of the vibration, with there being a significant main effect on Torque (ramp phase: F[ 2, 14] =75.021, p <.001, ηp²=0.915) ( Fig. 3A). These results were supported by a two-way repeated measure ANOVA with factor Vibration and Torque on the i EMG AGO. However, for the SS phases, i EMG AGO decreased with the vibration, especially at MVT 60 conditions (SS phase: F[ 1, 7] =12.340, p <.01, ηp²=0.638), which showed a significant factor interaction confirmed by the fact that the difference on i EMG AGO between the VIB conditions was observed only at the MVT 60 (SS phase: F[ 2, 14] = 9.913, p <.05, ηp²=0.421) ( Fig. 3B). These results are in the SS phase the i EMG RF ( i EMG RF: F[ 1, 7] =11.758, p <.01, ηp²=0.627) increased in VIB conditions, nevertheless the compensatory action of the remaining agonist muscles ( i EMG VL: F[ 1, 7] =36.177 p <.001, ηp²=0.838; i EMG VM: F[ 1, 7] = 21.535 p <.01, ηp²=0.755) caused a more significant decrease, and the overall i EMG AGO decreased statistically. Moreover, there was a significant effect of the vibration on i EMG ANT during SS phases. These observations have been supported by two-way repeated measure ANOVAs ( i EMG ANT: F[ 1, 7] =7.136, p <.05, ηp²=0.505). The CCI indicates the relative magnitude of i EMG ANT with respect to the overall muscle activation ( i EMG TOT). For both the ramp and SS phases, the CCI decreased with the torque magnitudes, while there was no significant difference between the VIB and NVIB conditions for all the torque conditions, which was confirmed by the results of a two-way repeated measure ANOVA with factor Torque and Vibration on the CCI. The main effect of Torque was significant without factor interaction (ramp phase: F[ 2, 14] =77.478, p <.001, ηp²=0.726; SS phase: F[ 2, 14] =38.506, p <.001, ηp²=0.847). Post-hoc pair-wise comparisons confirmed the CCI at MVT 20 >MVT 40 >MVT 60 for both the ramp and SS phases ( p <.05).
Fig. 3.
Fig. 3.All the variables are presented mean±standard error bar of i EMG in agonist muscles depending on all the torque conditions and the VIB & NVIB in (A) ramp & (B) SS phase. 
3. Multi-Muscle Coordination Pattern
To identify the effect of vibration on groups of muscles whose activity was modulated during the submaximal isometric ramp tasks. Three M-modes from five muscles using the i EMG signals. Table 1 shows the de-pendence of each average amount of variance explained by the three M-modes on vibration and torque conditions in the ramp and SS phases.
Table 1.
Total %Variance Explained by the Principal Components of ramp and SS phase
|
Level of Torque |
Ramp phase |
Steady-state (SS) phase |
|
VIB |
NVIB |
VIB |
NVIB |
|
PC1 M1-mode |
PC2 M2-mode |
PC3 M3-mode |
PC1 M1-mode |
PC2 M2-mode |
PC3 M3-mode |
PC1 M1-mode |
PC2 M2-mode |
PC3 M3-mode |
PC1 M1-mode |
PC2 M2-mode |
PC3 M3-mode |
|
MVT20
|
72.1 |
20.5 |
7.4 |
69.5 |
22.2 |
8.1 |
69.0 |
24.4 |
6.52 |
61.1 |
30.3 |
8.4 |
|
MVT40
|
75.1 |
24.0 |
0.8 |
74.6 |
21.3 |
4.0 |
67.1 |
30.2 |
2.60 |
60.9 |
34.7 |
4.3 |
|
MVT60
|
92.2
|
6.8
|
0.3
|
91.9
|
7.4
|
0.5
|
67.9
|
29.6
|
2.48
|
61.4
|
33.3
|
5.2
|
During the ramp phase, the percent variance explained by the three M-modes was no significant effect on the vibration. These results were supported by a two-way repeated measure ANOVA with factor Vibration and Torque on three M-modes with factor Vibration (2 levels: VIB and NVIB) and Torque (3 levels: MVT 20, MVT 40, and MVT 60), which showed a significant main effect of Torque for all M-modes (ramp phase: M 1-mode: F[ 2, 14] =6.55, p <.001, ηp²=0.484, M 2-mode: F[ 2, 14] =6.909, p <.01, ηp²=0.497, M 3-mode: F[ 2, 14] =55.22, p <.001, ηp²=0.887) while no factor interaction. However, for the SS phases, the M 1-mode (i.e., the most considerable variance of M-modes) was significantly larger in the VIB condition than in the NVIB condition ( F[ 1, 7] =21.047, p <.003, ηp²= 0.75). In particular, the VIB effect (i.e., increased percentage of M 1-mode with the vibration) was significant at MVT 20 and MVT 60 conditions, confirmed by posthoc pairwise comparisons ( p <.05) ( Fig. 4).
Fig. 4.
Fig. 4.All the variables are presented mean±standard error bar of Z-transformed variance explained in M1-mode (PC1) depending on all the torque conditions and the VIB & NVIB in SS phase. 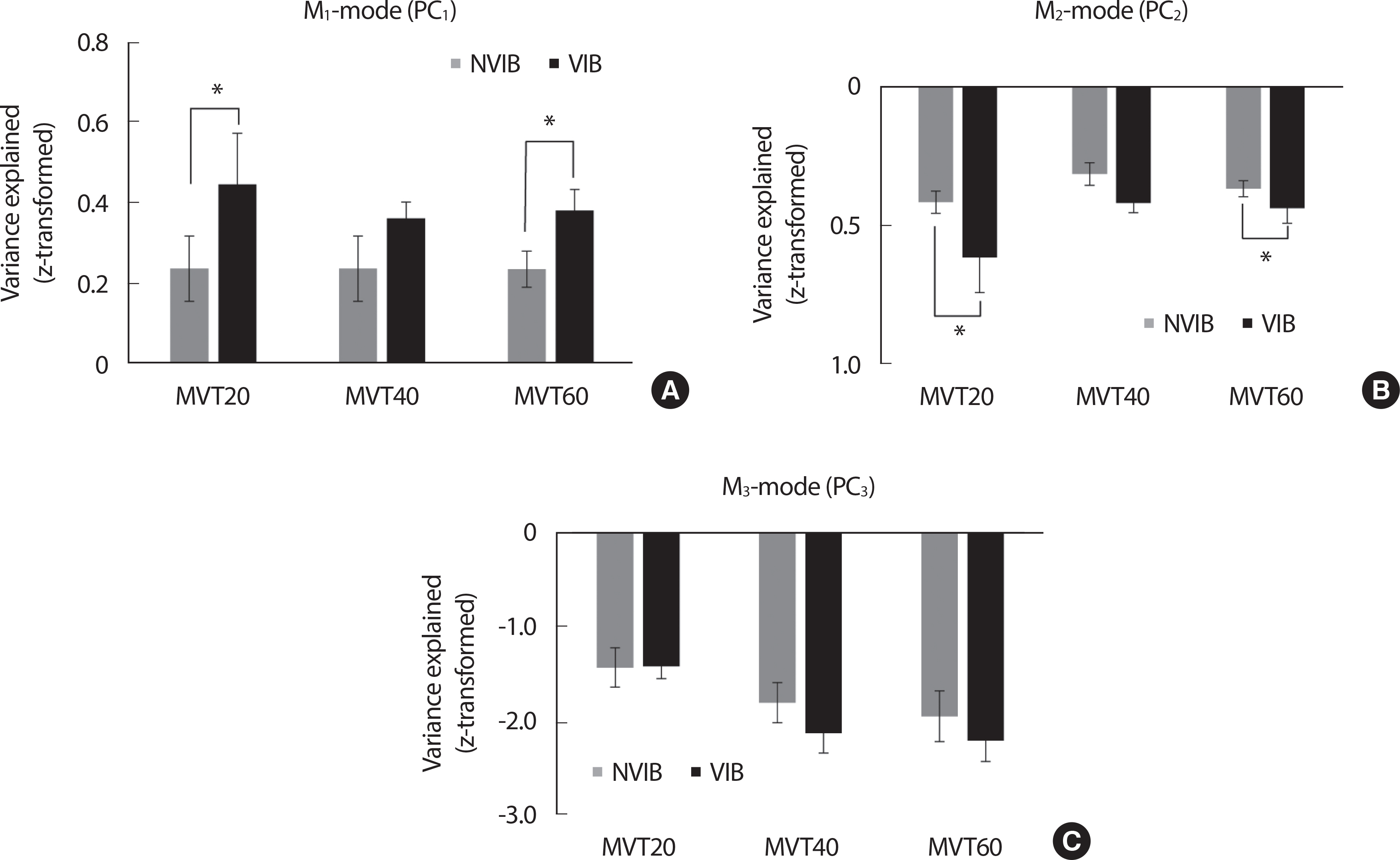
Table 2 shows the loading factors for all the muscles on the three M-modes for the ramp phase of MVT 60 in a representative subject. For the ramp phase of VIB and NVIB conditions, loading coefficients for all three M-modes at each individual muscle activation index were similar across subjects. This could be explained by muscle activation contributing to the reciprocal M-mode shown in Table 4.
Table 2.
Ramp phase loading coefficients for the PCA of a representative subject
|
Muscle |
VIB |
NVIB |
|
PC1
|
PC2
|
PC3
|
PC1
|
PC2
|
PC3
|
|
M1-mode |
M2-mode |
M3-mode |
M1-mode |
M2-mode |
M3-mode |
|
VM |
0.856
|
0.122 |
0.143 |
0.874
|
0.019 |
0.176 |
|
RF |
0.881
|
-0.065 |
0.034 |
0.854
|
-0.016 |
-0.018 |
|
VL |
0.861
|
0.076 |
0.172 |
0.870
|
0.027 |
0.168 |
|
BF |
0.334 |
-0.594
|
-0.726
|
0.300 |
-0.072 |
-0.946
|
|
ST |
0.105 |
-0.822
|
-0.559
|
0.004 |
-0.997
|
0.077 |
On the other hand, for the SS phase, the VIB condition of M 1-mode showed such typical cocontraction M-modes ( Table 3) [ 18]. However, in the NVIB condition of M1-mode a reduced cocontraction M-mode ap-peared. In particular, the loading magnitudes under <0.4 below in the RF without vibration, and the ST, which is the antagonist muscles.
Table 3.
SS phase loading coefficients for the PCA of a representative subject
|
Muscle |
VIB |
NVIB |
|
PC1
|
PC2
|
PC3
|
PC1
|
PC2
|
PC3
|
|
M1-mode |
M2-mode |
M3-mode |
M1-mode |
M2-mode |
M3-mode |
|
VM |
0.637
|
-0.132 |
-0.473
|
0.695
|
0.202 |
-0.114 |
|
RF |
0.488
|
-0.656
|
0.629
|
0.230 |
0.429
|
0.782
|
|
VL |
0.642
|
-0.312 |
-0.374 |
0.542
|
0.372 |
-0.480
|
|
BF |
0.718
|
0.268 |
0.173 |
0.449
|
-0.427 |
0.39 |
|
ST |
0.567
|
0.611
|
0.306 |
0.350 |
-0.714
|
-0.045 |
Table 4.
The muscles activated for the mode depending on phases and naming of each mode for PCA
|
Conditions |
Ramp |
SS |
|
VIB |
NVIB |
VIB |
NVIB |
|
M1-Mode |
VM, RF, VL
|
VM, RF, VL,
|
VM, RF, VL, BF, ST
|
VM, VL, BF
|
|
“Reciprocal M-mode” |
“Reciprocal M-mode” |
“Cocontraction M-mode” |
“Cocontraction M-mode” |
|
M2-Mode |
BF, ST
|
ST
|
RF, ST
|
RF, BF, ST
|
|
“Reciprocal M-mode” |
“Reciprocal M-mode” |
“Mixed M-mode” |
“Mixed M-mode” |
|
M3-Mode |
BF, ST
|
BF
|
VM, RF
|
RF, VL
|
|
“Reciprocal M-mode” |
“Reciprocal M-mode” |
“Mixed M-mode” |
“Mixed M-mode” |
DISCUSSION
The current results of the current study support both two hypotheses formulated in the introduction. First, RMSE NORM was reduced in MVT60 in both ramp and SS phases by the effect of vibration. Second, the M-mode composition (i.e., multi-muscle coordination pattern) was found to be different in VIB and NVIB in the SS phase. We found that the VIB condition showed a significantly larger cocontraction M-mode.
External vibration stimulation given to muscles stimulates the muscle spindles of intrafusal muscle fibers and activates primary afferent nerves at high levels. In particular, it is reported that the increased activity of these primary afferent nerves towards the spinal motoneuron can increase the membrane potential of the motoneuron without the contribution of additional central drives, leading to increased spontaneous force generation [ 28– 30]. Several previous studies that have applied vibration stimuli have shown an increase in force generation [ 31]. The maximal voluntary torque (MVT) capacity in our previous study also increased about 20% when vibration stimulation was applied to the rectus femoris [ 16]. In addition, similar to the task of this study, vibration stimulation improves the nervous system capacity, such as a specific receptor feedback mechanism, in a task that generates a constant target force while reducing the activity of the agonist without increasing the size and number of muscle fibers, thereby exhibiting positive impacts on the accuracy [ 32]. By combining the existing knowledge and the current results, the accuracy of task performance was increased (e.g., RMSE NORM decreased) in both ramp and SS phases by vibration stimulation given to the RF muscle at relatively high force demand, and iEMG was statistically significantly smaller in VIB condition than NVIB in the same torque level (e.g., MVT 60). Also, it was confirmed that the composition of M-mode showed relatively larger cocontraction M-modes due to vibration stimulation in the SS phase. These results indicate that a given vibration stimulus increased the force production efficiency of the agonist, especially at the MVT 60 condition, resulting in better performance accuracy. It has been proven that the accuracy of the task is improved by compensating the agonist torque using a cocontraction strategy; therefore, the reduction in agonist activity does not exceed the target level [ 33]. In addition, the mechanical action of vibration stimulation changes the muscle-tendon complex by sensory receptors, thereby increasing joint stiffness through the regulation of cocontraction between agonist and antagonist, which is reported as an advantageous human movement strategy to increase accuracy [ 34]. Our findings show that indices of muscle activity associated with the accuracy of the submaximal isometric ramp tasks can be described with a few principal components (i.e., M-modes). We suggest that this result support our second hypothesis that from muscle vibration, the CNS uses a few central variables to adjust the activity of the multi-muscles contrib-uting to the production of an accurate knee extension torque. In other words, the CNS uses a lower-dimensional space of control variables to produce changes in a higher-dimensional space of muscle activation. M-modes represent a combination of muscle activation that reduces the number of degrees of freedom manipulated by the CNS. It has been sug-gested that CNS has the ability to combine M-modes in different ways, depending on the situation, to achieve performance stability [ 35]. Consistent with these previous studies, we found significant differ-ences between the VIB condition in the M-modes configuration, even though the NVIB condition showed similar variances accounted for by three M-modes as the VIB condition. This finding showed that the VIB condition had more cocontraction M-modes controlling agonist and antagonist together than NVIB in the SS phase. There are fewer cases of significant cocontraction M-mode in the NVIB. Also, the results showed a tendency to increase CCI in the VIB condition compared to NVIB, and i EMG ANT did not show a statistically significant difference by vibration stimulation. The role of cocontraction is facilitating proprioception, particularly Ia afferent output, which may be beneficial in accuracy tasks [ 36, 37]. Nevertheless, it is reported that the mechanical advantage of cocontraction can vary depending on the task and system of interest. If the task is to increase task stability (i.e., accuracy), kinematic chains with fixed origin (e.g., sitting in a chair and the trunk was prevented from moving) are an advantage. In addition, cocontraction works advantage when considering neural control of related effectors that can guarantee movement stability beyond mechanical analysis [ 37]. There-fore, the increment of the cocontraction M-modes caused by vibration stimulation is assumed to be a control strategy to enhance the performance accuracy governed by both supraspinal and spinal levels (e.g., tonic vibration reflex) [ 38].
CONCLUSION
In conclusion, the results of the current study suggest that neurophysiological changes due to vibration stimulation can be positively influenced by task characteristics and force levels, which require the accuracy of torque generation. It will provide the basis for some quantitative understanding of the accuracy of exercise goals and the resulting multi-muscle coordination patterns of different muscle groups. Furthermore, vibration stimulation will be used as a tool for positive muscle response, contributing to training equipment for improving performance and injury prevention and rehabilitation techniques. Nevertheless, the apparent limitation of the current experiment is the relatively small cohort of subjects and no female composition. Future research will have to consider a large cohort of subjects and balanced composition of male-female in order to generalize the current claims.
REFERENCES
1. Eklund G, Hagbarth KE. Normal variability of tonic vibration reflexes in man. Exp Neurol. 1966;16(1):80-92.   2. Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3-7.   4. Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and golgi tendon organs. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2007;36(1):21-9.   5. Bianconi R, Van der Meulen JP. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963;26(1):177-90.   6. Brown MC, Engberg I, Matthews PB. Relative sensitivity to vibration of muscle receptors of cat. J Physiol-London. 1967;192(3):773-800.   7. Hagbarth K, Eklund G. Tonic vibration reflexes (TVR) in spasticity. Brain Res. 1966;2(2):201-3.   8. Herman R, Mecomber SA. Vibration-elicitkd reflexes in normal and spastic muscle in man. Am J Phys Med. 1971;50(4):169-83.  9. Park HS, Martin BJ. Contribution of the tonic vibration reflex to muscle stress and muscle fatigue. Scand J Work Env Hea. 1993;19(1):35-42.   11. Cordo PJ, Burke D, Gandevia SC, Hales JP. Mechanical, neural and perceptual effects of tendon vibration. Progress in motor control. 1998;1:151-71.
12. GoodwinGM MD, Matthews P. The contribution of muscle afferents to kinesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705-48.   14. Issurin V, Tenenbaum G. Acute and residual effects of vibratory stimulation on explosive strength in elite and amateur athletes. J Sports Sci. 1999;17(3):177-82.   15. Johnston RM, Bishop B, Haven Coffey G. Mechanical vibration of skeletal muscles. Phys Ther. 1970;50(4):499-505.   16. Lee J, Song J, Ahn J, Park J. The effect of short-term muscle vibration on knee joint torque and muscle firing patterns during a maximal voluntary isometric contraction. KJSB. 2017;27(2):83-90.  17. Latash ML, Gorniak S, Zatsiorsky VM. Hierarchies of synergies in human movements. Kinesiology. 2008;40(1):29.  19. Hislop H, Avers D, Brown M. Daniels and worthingham’ s muscle testing-e-book: techniques of manual examination and performance testing. Elsevier Sci 2013 Sep 27.
21. Lindahl O, Movin A, Ringqvist I. Knee extension: measurement of the isometric force in different positions of the knee-joint. Acta Orthop Scand. 1969;40(1):79-85.   22. Perotto AO. Anatomical guide for the electromyographer: the limbs and trunk. Charles C Thomas Publisher 2011 Aug 1.
28. Warman G, Humphries B, Purton J. The effects of timing and application of vibration on muscular contractions. ASEMCG. 2002;73(2):119-27.  29. Rehn B, Lidström J, Skoglund J, Lindström B. Effects on leg muscular performance from whole-body vibration exercise: a systematic review. Scand J Med Sci Sports. 2007;17(1):2-11.   30. Lebedev MA, Poliakov AV. Analysis of the interference electromyogram of human soleus muscle after exposure to vibration. Neurophys-iology. 1991;23(1):57-65.
31. Humphries B, Warman G, Purton J, Doyle TL, Dugan E. The influ-ence of vibration on muscle activation and rate of force development during maximal isometric contractions. J Sports Sci Med. 2004;3(1):16.   34. Gribble PL, Mullin LI, Cothros N, Mattar A. Role of cocontraction in arm movement accuracy. J Neurophysiol. 2003;89(5):2396-405.   35. Latash ML, Scholz JP, Schöner G. 28. Motor control. 2007;11(3):276-308.  36. Hulliger M, Dürmüller N, Prochazka A, Trend P. Flexible fusimotor control of muscle spindle feedback during a variety of natural movements. Prog Brain Res. 1998;80:87-101.
38. Gillies JD, Burke DJ, Lance JW. Supraspinal control of tonic vibration reflex. J Neurophysiol. 1971;34(2):302-9.  
|
|









