DEMENTIA AND MILD COGNITIVE IMPAIRMENT
In dementia, cognitive abilities like language, memory, and judgement are compromised, making it challenging to carry out daily tasks. In contrast, mild cognitive impairment (MCI), a pre-dementia stage, is charac-terized by decreased cognitive abilities but no restrictions on activities of daily living. MCI is a neurological disorder brought on by ageing [1]. As people age, their brains lose weight and volume, which causes about 50% of their nerves to die while still maintaining some of their partial neu-rons [2]. Additionally, the rate of progression to dementia is about 10-14% in MCI as opposed to 1-2% in the general elderly population, and visual perception ability declines three to four years prior to the onset of dementia [3]. It is quite high. In Korea, the number of dementia patients has roughly quadrupled over the past ten years, and it is predicted that 880,000 people aged 65 and over will have dementia in 2021, with a 10% dementia prevalence [4], and MCI will affect 1 in 5 seniors [5]. It is important to research the correct definition of mild cognitive impairment and suitable interventions to prevent dementia.
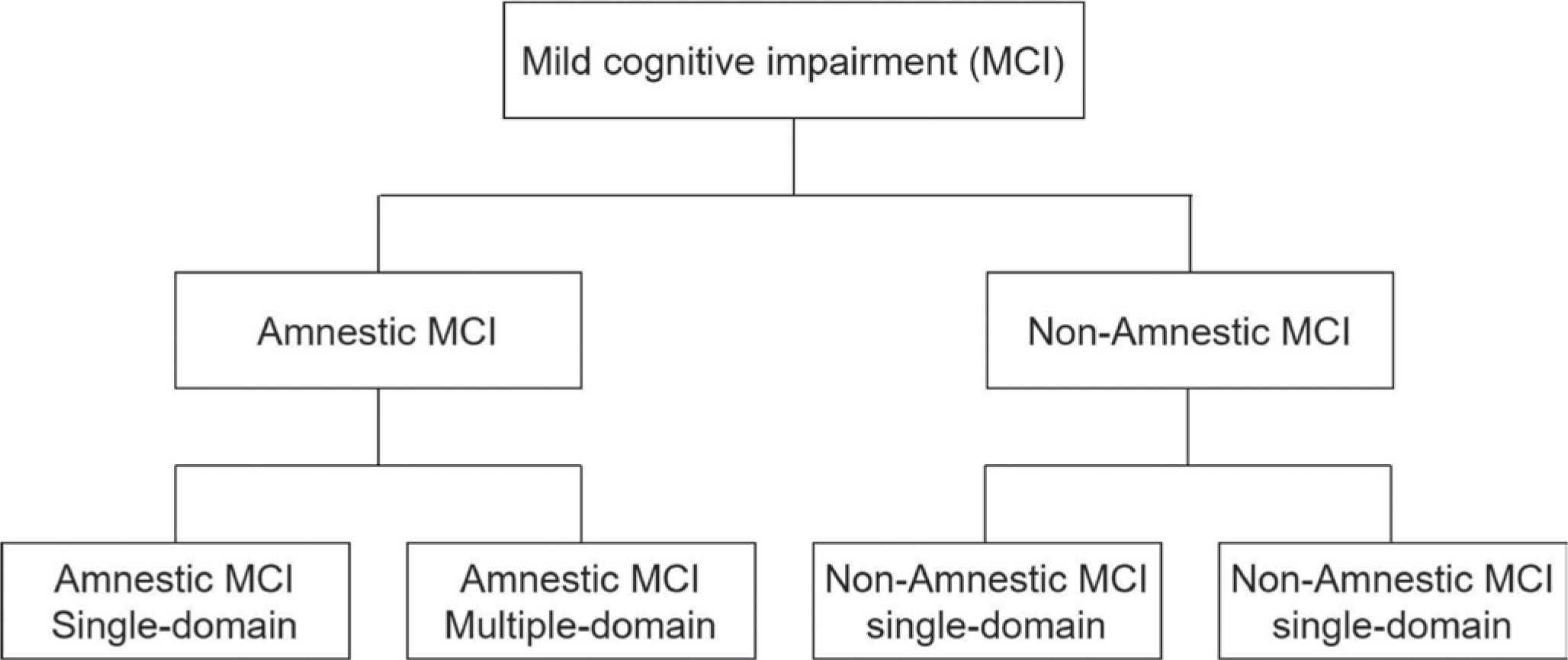
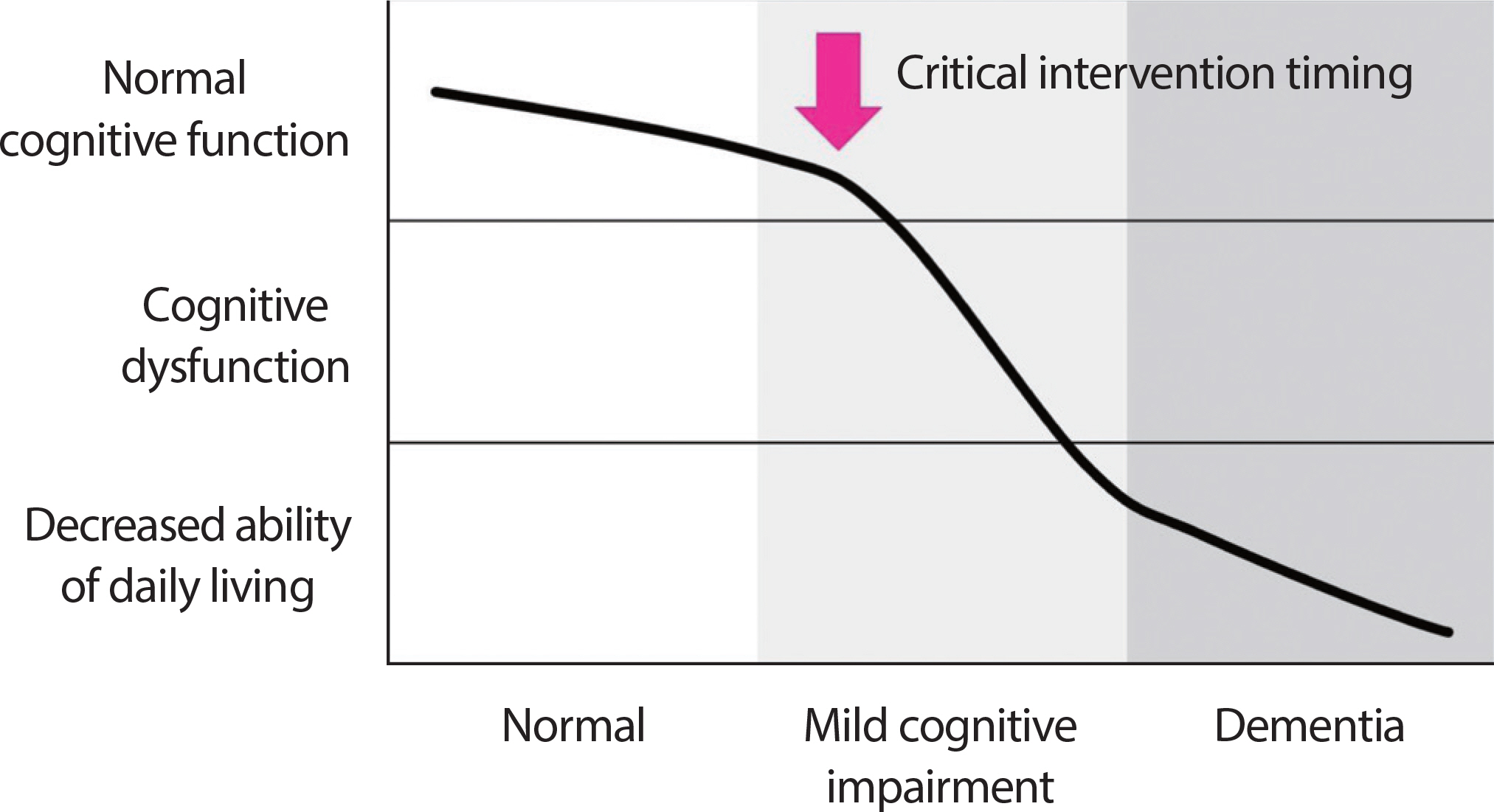
The criteria proposed by Petersen et al. [6] are primarily employed for the diagnosis of MCI, and they include subjective memory, objective memory based on age or educational level, overall cognitive function, maintenance of daily living activities, and non-dementia cases. According to the presence or absence of memory impairment, MCI is divided into amnestic type MCI (aMCI) and non-amnestic MCI (naMCI), and according to the number of cognitive domains in which impairments appear, they are classified into single-domain and multiple-domain (Fig. 1). The cognitive domains of memory and recall show a marked decline in aMCI, whereas the decline in other cognitive domains appears to be relatively close to normal or to a slight level. The majority of MCIs are amnestic MCI, which is a precursor to Alzheimer’ s. In addition, while naMCI has a relatively normal memory, its language, executive, and spa-tiotemporal abilities have all significantly declined, and its risk of developing non-dementia Alzheimer's is high. MCI is sometimes broken down into three categories: subjective memory impairment (SMI), early MCI (EMCI), and late MCI (LMCI) [7]. EMCI is defined as a standard deviation of the mean of normal cognitive function between 1.0 and 1.5, while LMCI is defined as a standard deviation of the mean of normal cognitive function less than 1.5. Additionally, SMI is a case of subjective-ly complaining of memory impairment with normal cognitive function and is very likely to progress to Alzheimer's disease. As a result, com-pared to other elderly people, MCI has a higher likelihood of developing into dementia, but there are no limitations on daily activities. As a result, it will be possible to delay the progression of dementia through appropriate diagnosis and intervention, which will have a positive impact on dementia prevention. It seems like a crucial period right now (Fig. 2).
Fig. 2.
Fig. 2.Critical intervention timing for the prevention of dementia in mild cognitive impairment.
While this was going on, the Korean Society for Dementia revealed that the “ People's Awareness Survey on Mild Cognitive Impairment” revealed that about 6 out of 10 people were unaware that MCI is a medical condition [8]. According to reports, there are numerous difficulties in the areas of diagnosis and insurance because it is regarded as a disease and is categorized as a F code in the code. This appears to be the outcome of the limited clinical diagnosis, treatment, and intervention for MCI that have been implemented thus far. As a result, it is believed that MCI awareness needs to be improved, and active management is required. In the future, an effectiveness-based management system for MCI will be necessary, as well as a social and national response to geriat-ric diseases. I consider convergence science research to be essential.
ROLE OF EXERCISE SCIENCE IN MILD COGNITIVE IMPAIRMENT
It is essential to approach medical issues and exercise physiology because MCI has a variety of clinical symptoms and causes. Cholinesterase inhibitors have been shown to slow the progression of Alzheimer's and dementia when used as medical drug treatments [9], and taking blood pressure medications, antiplatelet medications, and anticoagulants [10] as well as folic acid, vitamin B, and vitamin D [11] has been shown to slow the progression of dementia by controlling vascular risk factors. The administration of an acetylcholinesterase inhibitor to MCI, however, was reported to have caused side effects rather than slowing the progression of dementia [12,13].
However, regular exercise has been shown to reduce dementia risk factors by enhancing cognitive function for elderly task execution and increasing focus on hearing and vision [14]. Examining the mechanism of action of exercise, it is found that it stimulates nerve factors to promote nerve growth [15] and lowers the risk of cardiovascular and cere-brovascular diseases by ensuring that the brain receives blood and oxy-gen in a smooth manner [16]. As a result of increasing neuronal production, exercise ultimately has a positive impact on the improvement of cognitive function. It is well known that dementia patients experience a very rapid rate of hippocampus atrophy, which is a general decline in hippocampus size with ageing. As a result, consistent exercise increases the volume of the hippocampus and grey matter in the brain [17–19], and it also raises the blood level of brain-derived neurotrophic factor (BDNF), a protein that is essential for the development and differentiation of nerve cells. It has reportedly been shown to boost concentration [20]. Additionally, resistance exercise raises blood levels of insulin-like growth factor I (insulin-like), which is important for the growth and differentiation of nerve cells, and N-acetylaspartate (NAA), a chemical related to working memory [21]. There have been reports of an increase in growth factor I, IGF-I [22,23]. Numerous studies have shown a strong relationship between regular exercise and cognitive function in older people.
Exercise can generally be categorized into three types: low-intensity, medium-intensity, and high-intensity. Exercise of all intensities reduced cognitive decline by 38%, 35% for low-intensity and moderate-intensity exercise, and 35% for high-intensity exercise. Regular exercise also slowed cognitive decline [24]. Exercise duration is not always correlated with cognitive function [25], but for aerobic exercise, it is advised to exercise for at least 30 minutes each session [26], and for resistance exercise, it is advised to exercise twice per week [27]. High-intensity exercise is generally defined as being 80% or more of a person's 1 RM, while moderate-intensity exercise is defined as being 50% to 60% of 1 RM, or a weight that can be lifted for 8 to 15 reps (8-15 RM) corresponds to the degree. Consequently, it is advised to exercise for dementia at a moderate intensity [28]. Recently, one of the composite therapies that can help restore cognition is the dual task method, which has recently been used as a non-pharmacological dementia therapy. Performing two or more tasks simultaneously, or one task while working on another, is referred to as a “ dual task.” Exercise therapy and cognitive therapy must be used simultaneously or in conjunction with each other for MCI because the dual task activates a wider area of the brain, which is closely related to perfor-mance on two tasks [29,30]. For instance, moderate-intensity resistance exercise and cognitive training to stimulate cognition along with aerobic exercise and strength training for MCI, or it is necessary to continuously perform verbal tasks or to memorise numbers and letters backwards while exercising. However, since there are currently no appropriate exercise recommendations for MCI, exercise scientists must conduct in-depth research on compound exercise and exercise intensity. Because of this, it is challenging to demonstrate the effectiveness of drug therapy for MCI. In contrast, exercise anatomically alters the hippocampal volume of the brain, which in turn improves brain plasticity and cognitive function. Exercise physiology strategies for mild cognitive impairment and exercise convergence science research are needed to create a dementia-friendly society in the future. This will help prevent dementia. I consider science to be crucial to the field.
EXERCISE CONVERGENCE SCIENCE IN THE AGING SOCIETY
Worldwide, there is a sharp rise in the number of elderly people, and as a result of this, dementia-related infrastructure is also steadily expanding. A nationwide network of 256 dementia relief centres is supported by the Central Dementia Center in Korea, which also runs a number of dementia-related education programmes for those who work in facilities like the regional dementia centre and the dementia relief centres [31]. These programmes help to create and maintain the infrastructure that supports dementia services. When examining the infrastructure for dementia care in hospitals, cognitive therapy, exercise therapy, and psycho-therapy are the main treatments offered. When cognitive therapy is used, a computerised cognitive programme is used to give each client person-alised feedback, which helps to improve overall cognition, including memory, language, and recall. In addition, exercise therapy is used to promote falls and regular daily activities, as well as to reduce the rate at which dementia progresses through low-, moderate-, and high-intensity exercise. However, these treatments are already being used once dementia has been diagnosed, and there is a lack of infrastructure for support services and treatment for MCI. For MCI, the pre-dementia stage, an in-tegrated management system and related infrastructure must be estab-lished in the future.
Recent advances in wearable digital technology make it possible to track physical activity by gathering information on steps taken, calories consumed, and sleep patterns. Wearable digital technology has the ad-vantage of being able to gather and record data on the immediate envi-ronment or changes in physical activity. Wearable digital technology cannot stop ageing in this day and age, but it is anticipated that it will be possible to analyse the relationship between physical activity and aging-related diseases and related data, as well as to develop content that can be accessed through exercise physiology. Standardized evaluation criteria are required to stop the progression of MCI to dementia and to prevent dementia, especially because MCI is a critical stage in the treatment of dementia and a stage that may be a crucial step in preventing dementia. If activity analysis is used, it is believed that a reliable diagnosis will be possible. Additionally, it is anticipated that using wearable digital technology to look at aging-related biological indicators and an exercise physiology-based approach, individualized cognitive care for MCI patients will be possible. Finally, it is anticipated that exercise convergence science will make a significant contribution to exercise scientists’ research as well as the solution of MCI's social problems in the ageing population.