The Impact of Ballet Program on Appetite Related Hormones, Insulin Resistance, and Body Composition in Postmenopausal Women: A Pilot Study
Abstract
PURPOSE
Postmenopausal status is associated with an age-related decline in hormones, abnormal appetite regulation, weight gain, and increased risk of cardiovascular disease (CVD). Exercise is a useful non-pharmacological intervention that improves the levels of hormones involved in appetite regulation and weight management while reducing CVD risk factors. The present study aimed to elucidate the effect of a ballet program on menopause-related changes in satiety or levels of appetite regulation hormones, including leptin and ghrelin, homeostatic model of assessing insulin resistance (HOMA-IR).
METHODS
The EX group performed the ballet program. In the fasting state, body composition; leptin, ghrelin, insulin, and glucose levels; and HOMA-IR values were assessed before and 12 week after the intervention.
RESULTS
A significant group by time interaction (p<.05) was noted for percentage body fat, leptin, insulin, and HOMA-IR, which significantly decreased (p<.05), and ghrelin, which significantly increased (p<.05).
CONCLUSIONS
These results indicate that this ballet program may be an attractive and enjoyable intervention for improving meno-pause-associated appetite regulation hormone changes in postmenopausal women.
Keywords: Word, Menopause, Leptin, Ghrelin, Glucose, Insulin, HOMA-IR
INTRODUCTION
The cessation of menses, or menopause, generally occurs in women between the ages of 45 and 55. As the world's population continues to age, it is projected that by 2030 almost 1.2 billion women worldwide will be menopausal or postmenopausal [ 1]. Menopause is a process of aging that is associated with decreased estrogen production and has been previously associated with an increase in the development of cardiovascular disease (CVD) and metabolic disorders [ 2– 4]. Leptin and ghrelin play a critical role in the regulation of energy, food uptake, and body weight [ 5]. Leptin and ghrelin have previously been positively and negatively correlated with estrogen, respectively, which is known to decrease following menopause [ 6]. Alterations in levels of leptin and ghrelin have been previously linked to increases in appetite, obesity, and insulin resistance [ 6– 11]. The reduced insulin response results in a slower metabolism and reduces anabolic activity which con-tributes to weight gain [ 12]. In conjunction with these hormonal changes, the decreased energy expenditure previously reported in postmenopausal women [ 13], may explain the increase in central adiposity observed in postmenopausal women [ 7]. The hormonal and body composition changes that coincide with menopause leave postmenopausal women at risk of increased CVD [ 14– 17]. Previous research has shown that exercise is a useful non-pharmaco-logical therapy for weight management as it has been shown to positively affect appetite regulation [ 18], energy homeostasis hormones [ 19], and improve body composition [ 20, 21]. We have previously shown that exercise can also improve vascular and muscular function associated with the age-related decline in hormone production [ 22– 24] and reduce risk factors for CVD [ 21]. However, many older adults regard it difficult to participate in the exercise due to perceived barriers (e.g., expense, exercising safely, absence of good supervision, concerns about exercising safely) [ 25– 27]. Hence, it may be advantageous to offer an exercise modality that is attractive to older adults, specifically, postmenopausal women. Ballet can easily be modified to accommodate the preferences of individuals making it a style of exercise that may be more attractive to older adults. Ballet programs, such as dance, gymnastics, and ballet, can be programmed to incorporate components of both aerobic and anaerobic training [ 28– 31]. Although, the impact of ballet program on appetite-re-lated hormones, insulin resistance, and body composition in postmenopausal women remain largely unexplored. We hypothesized of this pilot study that the ballet program would exhibit improve levels of leptin, ghrelin, and HOMA-IR in postmenopausal women.
METHODS
1. Participants
Twenty postmenopausal women (age, 51-55 years) were recruited from Busan, South Korea. All participants were considered postmenopausal (≤1 year, cessation of menses), classified as obese (body fat percentage >30%) [ 32], and were considered inactive (i.e., ≤60 min/day, ≤1 days/week, at least the last 6 months) [ 33]. All participants were free of medi-cation use or hormone therapy during the year prior to the beginning of the study. Participants have not participated in regular exercise or weight loss program in the last 1 year and have not taken fat loss supplements. All participants gave their informed consent for inclusion before they participated in the study.
2. Study design
The present study utilized a two-armed parallel experimental design. All blood samples and body composition were measured pre and post the 12-week ballet program. Baseline measurements were performed at 8:00 AM (±1 hour) after an overnight fast. Following baseline assess-ment, participants were randomly assigned to either the ballet program group (EX, n=10) or the control group (CON, n=10) with computer-generated random numbers. Laboratory personnel were not aware of the allocation. The ballet program took place April through July. The participants in the EX-group performed ballet program 60 minutes per day, 3 days a week for 12 weeks. Ballet program was performed Monday, Wednesday, and Friday each week at 11:00 AM (±1 hour) and consisted of variations of ballet dance maneuvers.
3. Blood sample analysis
Plasma Leptin concentrations were assessed using a Human Leptin RIA kit (LINCO Research, USA) and COBRA 5010 Quantum (PACKARD, USA) [ 34]. Plasma ghrelin concentrations were assessed using commercial RIA kits (LINCO Research, USA) and COBRA 5010 Quantum (PACKARD, USA) [ 35]. Serum insulin was evaluated using an electrochemiluminescence immunoassay (Roche, Germany) and cobas ®8000 modular analyzer series (COMPANY, Roche, Germany). The serum glucose was evaluated with a glucose UV spectrophotometry immunoassay (COMPANY, Roche, Germany) and cobas ®8000 modular analyzer series (COMPANY, Roche, Germany). HOMA-IR was a calculation by using the ensuing formula: HOMA-IR=[fasting glucose (mg/dL)× fasting insulin (μU/mL)/405] [ 36].
4. Body composition.
Height and body mass were measured to the nearest 1.0 cm and 0.1 kg. %BF and lean body mass were measured to the nearest 0.1 kg, per-cent body fat was measured to the nearest 0.1%, and lean body mass (LBM) were measured to the nearest 0.1 kg using bioelectrical imped-ance analysis (BIA) (X-SCAN PLUS II, Korea) [ 37].
5. Ballet program
Participants in the EX group performed the ballet program for 12 weeks. Participants performed the ballet program on Mondays, Wednes-days, and Fridays every week and each session was 60 minutes in duration ( Table 1). Each class began with a 10-minute warm-up that consisted of abdominal breathing and stretching that emphasized the preparation of major muscle groups for exercise. The main exercise component of each class was followed by a 10-minute cool-down.
Table 1.
|
Order |
Exercise |
Duration |
Intensity |
Frequency |
|
Warm-up |
Abdominal breathing & stretching |
10 min |
|
|
|
Main Exercise |
1. Pliés, 2. Battement tendus, |
40 min |
1-6 weeks |
3 times/week |
|
3. Battement jetés, 4. Adagio |
|
HRR: 50-60% |
|
|
5. Grand battements, |
|
|
|
|
6. Adagio-center |
|
|
|
|
7. Battement tendu-center, |
|
|
|
|
8. Grand battement -center |
|
|
|
|
9. Small jump: Temps soute-center, |
|
|
|
|
10. Pas glissade |
|
|
|
|
1. Pliés, 2. Battement tendu+Releves |
|
7-12 weeks |
|
|
3. Battement jetés+piques, |
|
HRR: 60-70% |
|
|
4. Rond de jamb parterre, |
|
|
|
|
5. Adagio, 6. Grand battement, |
|
|
|
|
7. Adagio: Pliés+port de bras, |
|
|
|
|
8. Battement tendu+Turn, |
|
|
|
|
9. Grand battement -Grand turn, |
|
|
|
|
10. Small jump: Changement de pieds, |
|
|
|
|
11. Pas assemble, 12. Petit pas chasse |
|
|
|
|
13. Grand pas jetés |
|
|
|
|
Cool-down |
Arabesque, Glissade, Waltz, |
10 min |
|
|
|
Changement Reverence |
|
|
|
The ballet program intensity was determined using heart rate reserve (HRR). Intensity of ballet program was gradually increased from 50% to 60% HRR in weeks 1 to 6, and to 60% to 70% HRR in weeks 9 to 12 [ 38]. During each ballet program session subjects wore a Polar chest heart rate monitor to monitor the exercise intensity (Polar, RS400sd, APAC, USA). Each ballet class was supervised by qualified researchers.
6. Statistical analysis
Data are expressed as Mean±Standard Deviation (M±SD). Indepen-dent t-tests were used to examine baseline differences between the two groups. A two-way analysis of variance with repeated measures [group (EX and CON)×time (pre-and post- 12 weeks) was used to examine the difference of changes between before and after ballet program within and between groups. Bonferroni correction was used to determine the effects of ballet program over time. When significant interaction were noted, paired t-tests were used for post-hoc comparisons. Data were ana-lyzed using SPSS 25 (SPSS Inc. Chicago, IL, USA). unless noted other-wise. Statistical significance was set to p <.05. The sample dimension analysis was performed using G*Power software (GPower 3.1). A total sample size of 10 subjects was required to satisfy a significance level of 0.05 with effect size of 0.99 and power of 0.80.
RESULTS
Baseline measurements were not significantly different between the CON and EX groups ( p >.05) ( Table 2). Following the 12-week ballet program there were significant group x time interactions ( p <.05) for %BF, leptin, ghrelin, insulin, and HOMA-IR. No significant changes were observed in the CON group ( p>.05). Following the ballet program, concentrations of ghrelin ( Fig. 1) significantly improved ( p <.05) in the EX group and %BF ( Table 2), leptin ( Fig. 2), insulin ( Fig. 3B), and HOMA-IR ( Fig. 3C) were significantly decreased ( p<.05) in the EX group.
Fig. 1.
Fig. 1.Ghrelin pre and post 12 weeks of control group and exercise group in postmenopausal women. Values are presented as Mean±SD. * p<.05 vs. Pre; † p<.05 vs. CON. 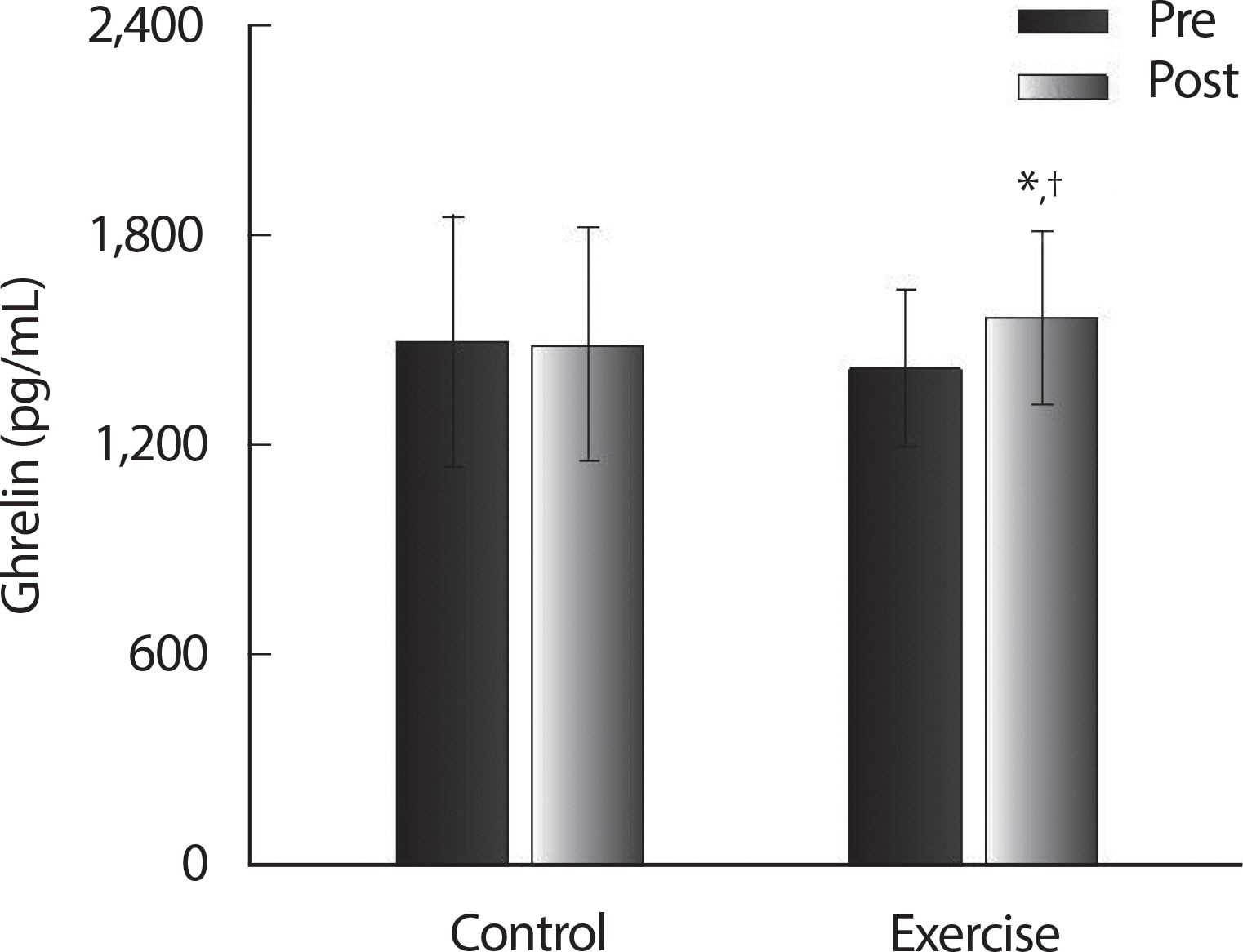
Fig. 2.
Fig. 2.Leptin pre and post 12 weeks of control group and exercise group in postmenopausal women. Values are presented as Mean±SD. * p<.05 vs. Pre; † p<.05 vs. CON. 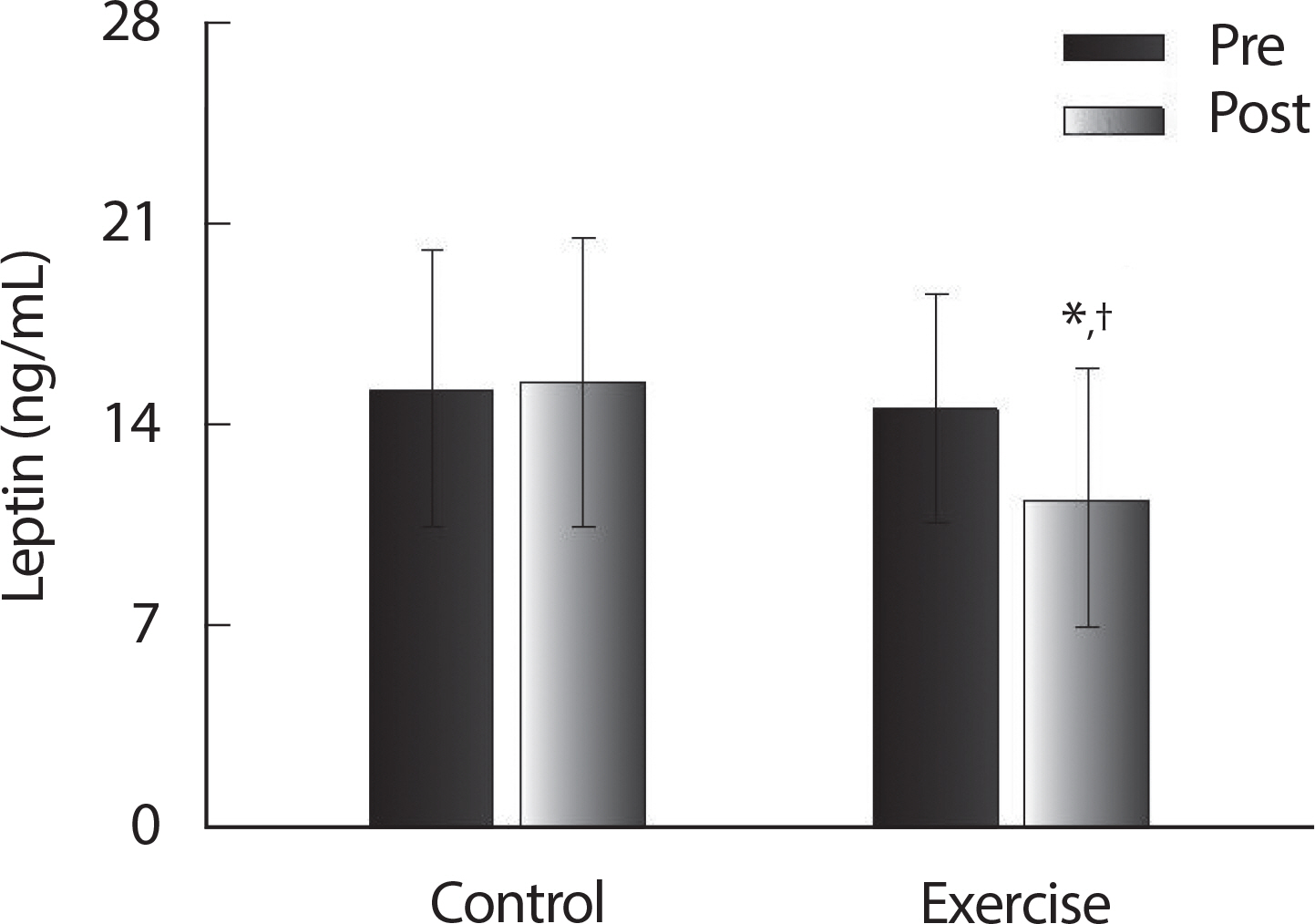
Fig. 3.
Fig. 3.Glucose (A), insulin (B) and HOMA-IR (C) pre and post 12 weeks of control group and exercise group in postmenopausal women. Values are presented as Mean±SD. * p<.05 vs. Pre; † p<.05 vs. CON. 
Table 2.
Descriptive characteristics of the study participants
|
CON (n=10) |
EX (n=10) |
|
Pre |
Post |
Δ |
Pre |
Post |
Δ |
|
Age (yr) |
52.1±2.47 |
- |
- |
52.9±1.85 |
- |
- |
|
Height (cm) |
159.6±5.14 |
- |
- |
159.7±4.95 |
- |
- |
|
Weight (kg) |
60.59±5.86 |
60.65±6.72 |
0.06±0.36 |
60.63±4.20 |
58.53±4.07 |
-2.10±0.13 |
|
BMI (kg/m2) |
23.84±2.69 |
23.86±2.64 |
0.02±0.13 |
23.81±1.89 |
22.32±1.48 |
-0.99±0.59 |
|
Body fat (%) |
31.54±3.03 |
31.78±3.75 |
0.24±0.72 |
31.31±1.53 |
29.09±1.33 |
-2.22±0.20*,†
|
|
Lean body mass d=0.69 |
23.1±2.04 |
22.86±1.79 |
-0.24±0.25 |
22.78±1.20 |
23.86±0.98 |
1.08±0.22*,†
|
DISCUSSION
1. Ghrelin
Ghrelin is known as the hormone that is secreted by enteroendocrine cells within the gastrointestinal tract [ 39]. In this present study, participants body mass was significantly decreased in the EX group. In obese individuals with increased circulating levels of leptin, the level of ghrelin is decreased [ 5]. Ghrelin levels are associated with balance between energy expenditure and food intake [ 5]. A previous study reported that with decreased fat mass, ghrelin levels are increased [ 40]. This may be the bodies response to an increase in energy expenditure. The subjects in the ballet program group experienced an improvement in %BF and LBM. Ghrelin has also been positively associated with adiponectin levels in postmenopausal women [ 7]. This hormone plays an important role in increased glucagon secretion, osteoblast proliferation/differentiation, and can protect against muscle atrophy [ 41]. Increasing ghrelin levels may at first seem counterintuitive, however it has been shown that this increase in obese postmenopausal women contains anti-inflammatory properties [ 42– 44]. Along with these properties, increased ghrelin levels have been shown to regulate glucose homeostasis through inhibition of insulin secretion and regulation of hepatic glucose output [ 41]. With an increase in ghrelin the ballet program may help postmenopausal women regulate glucose more efficiently.
2. Leptin
A previous study reported that low levels of leptin in obese middle-aged women are associated with an increase of obesity [ 5]. Leptin is the hormone responsible for regulating energy balance by inhibiting hunger [ 45]. It has been previously reported that leptin plays a role in regulating of body weight and food intake [ 5]. This may reduce the progression and/or prevalence of obesity in this population. Leptin levels have also been shown to be influenced by the amount BF and LBM [ 46]. We found that 12 weeks of ballet program reduced %BF and increase LBM which may positively influence on leptin level in these individuals. Low-er estrogen has been shown to associated with lower leptin sensitivity [ 47]. In general, low estrogen levels are reported in postmenopausal women. Additionally, leptin level and leptin sensitivity are lower com-pared to premenopausal women. This lower leptin sensitivity may lead to increased hunger which may lead to obesity. Therefore, the improved body composition by the 12-week ballet program may have significantly influence the levels of leptin or vice versa. This result also consistent with the other results that Exercise lowers leptin concentrations caused by the appetite control and energy balance [ 48, 49]. However, we have not directly assessed the relationship between the reduction of leptin and body composition, and further investigation is warranted.
3. Insulin resistance
It is well understood that insulin resistance occurs when cells in the body cannot utilize insulin to uptake the increased glucose in the blood [ 50– 53]. Menopause and increases in abdominal adiposity result in de-creases in estradiol and increases in resistin, respectively, both of which have been shown to contribute to insulin resistance [ 50, 51, 54]. In addition, postmenopausal women typically show an increase in adiposity and will also generally be less active, which may explain why insulin resistance is so prevalent in obese middle-aged women [ 53]. Although it has been previously shown that women who are more physically active have greater insulin sensitivity, whether they are on hormone replacement therapy or not [ 53]. In the present study, within the exercise group, HOMA-IR decreased significantly versus the control. With the intro-duction of the ballet program, muscles that most likely have not been used so often are activated. Putting that demand on the musculature will force a greater need for glycogen. As exercise is completed, the body's glucose metabolism will be improved, and the capillarization of skeletal muscles will increase. These factors combined could potentially lead to higher insulin sensitivity and efficient glucose utilization. We demonstrate that insulin resistance levels in postmenopausal women improve following a 12-week ballet program, and the reduced insulin levels may be played a part in the improvement of insulin resistance. Glucose levels did not change; thus, it can be stated that the reduced HOMA-IR we have shown is predominantly due to reduced insulin levels following ballet program. Therefore, decreasing the levels of insulin resistance via the ballet program may reduce fat mass in this population.
4. Body composition
In this study, those who participated in the ballet program saw an increase in LBM, with a reduction in weight and body fat percentage. Postmenopausal women generally experience an increase in body fat percentage due to hormonal changes, aging, and reductions in physical activity [ 55, 56]. With an increased body fat percentage can come complications such as, diabetes and cardiovascular disease [ 57]. Therefore, an exercise program that is low impact, attractive and cost-efficient may be the answer for improving menopause-associated increases in body fat percentage. With improved body composition the subjects BMI also was decreased. As stated previously, participants experienced an increase in insulin sensitivity. This can most likely be attributed to the decrease in body fat percentage and increase in lean body mass. Insulin sensitivity can play an important role in this population. Previous studies have shown a strong influence of body fat percentage on insulin sensitivity in postmenopausal women [ 50– 53]. Others also report that menopause and aging are each associated with a reduction in insulin sensitivity [ 53]. Although we did not obtain food logs during the ballet program, we have supervised them not to change their regular diet habits and maintain their caloric intake. Therefore, reductions in total mass (body weight) within the ballet group may be attributed to an increase in energy expenditure. A reduction in body fat can be associated with increased leptin levels and leptin sensitivity [ 53]. This may potentially prevent our subjects not to consume extra caloric intake.
CONCLUSION
The results of this study demonstrated that the ballet program is a beneficial intervention in improving levels of leptin, ghrelin, insulin, and insulin resistance while reducing body fat in postmenopausal women. Although ghrelin levels were elevated in the ballet program group, exercise may have increased leptin sensitivity. These improvements in hormones are associated with reduced fat mass and increase lean mass in postmenopausal women. Thus, these results evidence that a ballet program may be an effective therapeutic intervention for the prevention of obesity in postmenopausal women.
REFERENCES
1. Research on the menopause in the 1990s. Report of a who scientific group. World Health Organ Tech Rep Ser.. 1996;866:1-107.  2. Rosano GM, Vitale C, Marazzi G, Volterrani M. Menopause and cardiovascular disease: the evidence. Climacteric.. 2007;10(Suppl 1):19-24.   3. Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, et al. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas.. 2001;39:125-32.   5. Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev.. 2007;8:21-34.   10. Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, et al. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol.. 2003;149:331-5.   11. Purnell JQ, Weigle DS, Breen P, Cummings DE. Ghrelin levels corre-late with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. J Clin Endocrinol Metab.. 2003;88:5747-52.   12. Coyoy A, Guerra-Araiza C, Camacho-Arroyo I. Metabolism regulation by estrogens and their receptors in the central nervous system before and after menopause. Horm Metab Res.. 2016;48:489-96.   14. Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, et al. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr Metab Cardiovasc Dis.. 2015;25:495-502.   15. Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Asso-ciation of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation.. 1999;99:541-5.   16. Jansen MA, Uiterwaal CS, Visseren FL, van der Ent CK, Grobbee DE, et al. Abdominal fat and blood pressure in healthy young children. J Hypertens.. 2016;34:1796-803.   17. Saijo Y, Kiyota N, Kawasaki Y, Miyazaki Y, Kashimura J, et al. Relation-ship between c-reactive protein and visceral adipose tissue in healthy japanese subjects. Diabetes Obes Metab.. 2004;6:249-58.   18. Vatansever-Ozen S, Tiryaki-Sonmez G, Bugdayci G, Ozen G. The effects of exercise on food intake and hunger: relationship with acylated ghrelin and leptin. J Sports Sci Med.. 2011;10:283-91.   20. Seo DI, Jun TW, Park KS, Chang H, So WY, et al. 12 weeks of combined exercise is better than aerobic exercise for increasing growth hormone in middle-aged women. Int J Sport Nutr Exerc Metab.. 2010;20:21-6.   21. Son WM, Sung KD, Cho JM, Park SY. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause.. 2017;24:262-8.   22. Ha MS, Son WM. Combined exercise is a modality for improving insulin resistance and aging-related hormone biomarkers in elderly ko-rean women. Exp Gerontol.. 2018;114:13-8.   23. Lee SH, Scott SD, Pekas EJ, Lee S, Lee SH, et al. Taekwondo training reduces blood catecholamine levels and arterial stiffness in postmenopausal women with stage-2 hypertension: randomized clinical trial. Clin Exp Hypertens.. 2019;41:675-81.   24. Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH. combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause.. 2011;18:980-4.   25. O'Neill K, Reid G. Perceived barriers to physical activity by older adults. Can J Public Health.. 1991;82:392-6.  26. Martin C, Woolf-May K. The retrospective evaluation of a general practitioner exercise prescription programme. J Hum Nutr Diet Suppl.. 1999;12:32-42.  27. Murphy S, Raisanen L, Moore G. The evaluation of the national exercise referral scheme in wales. Cardiff: Welsh Government 2010.
28. Wyon MA, Abt G, Redding E, Head A, Sharp NC. Oxygen uptake during modern dance class, rehearsal, and performance. J Strength Cond Res.. 2004;18:646-9.   29. Wyon MA, Redding E. Physiological monitoring of cardiorespiratory adaptations during rehearsal and performance of contemporary dance. J Strength Cond Res.. 2005;19:611-4.   30. Angioi M, Metsios GS, Koutedakis Y, Wyon MA. Fitness in contemporary dance: a systematic review. Int J Sports Med.. 2009;30:475-84.   31. Wanke EM, Quarcoo D, Uibel S, Groneberg DA. Rehabilitation after occupational accidents in professional dancers: advice with due regard to dance specific aspects. Rehabilitation (Stuttg).. 2012;51:221-8.  32. Brozek J, Kihlberg JK, Taylor HL, Keys A. Skinfold distributions in middle-aged american men: a contribution to norms of leanness-fat-ness. Ann N Y Acad Sci.. 1963;110:492-502.   34. Ostlund RE Jr, Yang JW, Klein S, Gingerich R. Relation between plas-ma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab.. 1996;81:3909-13.   35. Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, et al. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab.. 1997;82:4270-3.   41. Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care.. 2013;16:619-24.   43. Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappab activation in human endothelial cells. Circulation.. 2004;109:2221-6.   47. Hong SC, Yoo SW, Cho GJ, Kim T, Hur JY, et al. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause.. 2007;14:835-40.   48. Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev.. 2015;16:67-76.   50. Behboudi-Gandevani S, Ramezani Tehrani F, Cheraghi L, Azizi F. Could ‘a body shape index’ and ‘waist to height ratio’ predict insulin resistance and metabolic syndrome in polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol.. 2016;205:110-4.   51. Colman E, Katzel LI, Rogus E, Coon P, Muller D, et al. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism.. 1995;44:1502-8.   52. Villareal DT, Holloszy JO. Effect of dhea on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA.. 2004;292:2243-8.   56. Teede HJ, Lombard C, Deeks AA. Obesity, metabolic complications and the menopause: An opportunity for prevention. Climacteric.. 2010;13:203-9.   57. Albu JB, Kovera AJ, Johnson JA. Fat distribution and health in obesity. Ann N Y Acad Sci.. 2000;904:491-501.  
|
|








