Cho, Lee, and Seo: Effects of Exercise Sequence and Circadian Rhythms on Molecular Mechanisms of Muscle Hypertrophy and Mitochondrial Biogenesis in Obese Rat
Abstract
PURPOSE
This study aimed to investigate whether endurance and resistance training sequences and circadian rhythms affect muscle hypertrophy and mitochondrial biogenesis-related molecules in obese rats.
METHODS
Rats were randomly divided into five groups: the obesity control group (OCG), aerobic-resistance exercise in the morning group (ARMG), resistance-aerobic exercise in the morning group (RAMG), aerobic resistance exercise in the evening group (AREG), and resistance-aerobic exercise in the evening group (RAEG). The exercise groups performed endurance and resistance exercises for 8 weeks according to their circadian rhythms and exercise sequences.
RESULTS
We evaluated specific muscle hypertrophy and mitochondrial biogenesis markers in the flexor pollicis longus and soleus muscles using western blot and immunofluorescence techniques. The body weight was significantly decreased in the ARMG, RAMG, AREG, RAEG compared to the OCG after intervention. The soleus muscle fiber cross-sectional area and muscle hypertrophy markers p-Akt and p-mTOR were significantly increased in the AREG group compared with those in the OCG, ARMG, RAMG, and RAEG groups after exercise intervention. Most mitochondrial biogenesis-related markers were significantly increased in the RAMG group than in the other groups after exercise intervention.
CONCLUSION
Our findings provide new evidence that muscle hypertrophy might be upregulated by resistance exercise after evening endurance exercise. In addition, morning resistance exercise followed by aerobic exercise, might promote mitochondrial biogenesis.
Keywords: Circadian rhythms, Exercise sequence, Muscle hypertrophy, Mitochondrial biogenesis, Interference effect
INTRODUCTION
Obesity is an emerging global problem that promotes the incidence of chronic diseases such as cardiovascular disease, diabetes, and cancer [ 1]. Aerobic and resistance exercise is the most economical therapeutic method for preventing and managing obesity [ 2]. Regular aerobic exercise increases the expression of peroxisome pro-liferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and which are a transcription coactivator that regulates the generation and function of mitochondria and is closely related to metabolic regulation [ 3]. Also, aerobic exercise was upregulating the expression of 5’ adenosine monophosphate-activated protein kinase (AMPK), which are regulate mitochondrial biogenesis the adenosine monophosphate/adenosine tri-phosphate balance and PGC-1α activity [ 4]. Resistance exercise has been known to increase protein synthesis and muscle mass through the phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt)- mammalian target of rapamycin (mTOR) signaling pathway in human and animal models, which enhancement of muscle hypertrophy and muscle mass may be an important factor in improving sleep quality and various metabolic diseases [ 5]. Previous mechanism studies have reported that AMPK inhibits the expressed mTOR mole-cule. This interference effect emphasizes the importance of aerobic and resistance exercise sequence [ 6]. However, Ogasawara et al. [ 7] reported that Akt and mTOR phosphorylation levels did not differ with the exercise sequence. Therefore, it is necessary to reconfirm the mechanism of the mTOR signaling pathway. Recent study reported that circadian rhythms is a controllable therapeutic strategy that can improve obesity [ 8, 9]. The circadian rhythms are governed by in the suprachiasmatic nucleus, which is in the hypothalamus of the brain [ 10]. These circadian rhythms are closely related to sleep, heart rate, stress, metabolism function, hormones, and exercise capacity [ 11]. A previous study reported that the secretion of neurotrans-mitters and muscle function were improved in the afternoon exercise compared to in the morning exercise [ 12, 13]. In contrast, Kim et al. [ 14] reported that show no difference in lipid oxidation between morning and evening exercise. Considering these previous findings, obesity is a closely related to circadian rhythm and exercise sequence. However, research on muscle hypertrophy and mitochondrial biogenesis-related molecules in obesity according to the circadian rhythm and exercise sequence intervention are still insufficient. Therefore, the purpose of this study investigating whether the circadian rhythm and exercise sequence intervention on regulates muscle hypertrophy and mitochondrial biogenesis-related signaling pathway in fast and slow twitch fibers.
METHODS
1. Experimental animals
Female Sprague-Dawley rats (5 weeks old, female, n=30) were maintained on a high-fat diet (carbohydrates 20 Kcal%, protein 20 Kcal%, fat 60 Kcal% feed; D12492, Rodent Diet Inc., NJ, USA) for 10 weeks to in-duce obesity. The rats were considered obese if their body weight increased by 20% or more compared to the body weight of rats that were fed and water ad libitum the general diet (Commercial rat Chow, Samy-ang Co., Korea) [ 15]. The experimental rats were randomly divided into five groups: the obesity control group (OCG, n=6), aerobic-resistance exercise in the morning group (ARMG, n=6), resistance-aerobic exercise in the morning group (RAMG, n=6), aerobic-resistance exercise in the evening group (AREG, n=6), and resistance-aerobic exercise in the evening group (RAEG, n=6). Animals were maintained at a constant room temperature of 22-24° C and 60% of humidity under 12/12-hour light-dark cycle. This experiment obtained approval by the Ethics Com-mittee of Jeju National University (2021-0052).
2. Endurance and resistance exercise protocols
To adapt the treadmill walking, all rats in this study performed low intensity treadmill exercise for a week before the study began. The animals in the exercise groups were subjected to endurance running at a speed of 14-16 m/min for 30 minutes, three a week with no inclination by using Treadmill device (Jeungdo Bio & Plant). Also, there was the resistance exercise (ladder-climbing), the rats climbed a ladder with a length of 1 m, interval of 2 cm, and angle of 80°, three a week. The initial load was started at 50% of the animal body weight and gradually increased to 75%, 90%, and 100%. The rest time was set to 1 minute, and the exercise was continued until 8 climbs were completed or the entire length of the ladder could not be climbed. The weight was fixed to the proximal end of the rat tail using a swivel and tape. The exercise intervention was applied according to the exercise sequence and circadian rhythms in the rat (07:00 AM, 07:00 PM) for 8 weeks. The endurance and resistance exercise protocols were applied based on Zhang et al. [ 16], Cassilhas et al. [ 17] and Kraemer et al. [ 18] studies.
3. Western blot analysis
The dissected flexor pollicis longus and soleus muscles tissues were rinsed with phosphate-buffered saline (PBS) and lysed in Triton lysis buffer. Being denatured proteins were separated on sodium dodecyl sul-phate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane on ice at 200 mA for 2 hours. The membranes were blocked with 5% skim milk, 0.1% Tween 20 in tris buffered saline for 30 minutes at room temperature. Then, the membranes were incubated overnight with primary antibodies at 4° C. Protein (20 μg) was used for Western blot analysis using anti-Insulin like growth factor 1 (IGF-1) mouse polyclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-PI3K mouse polyclonal antibody (1:1,000, Cell Signaling Biotechnology, Danvers, MA, USA), anti-phosphorylated mTOR rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-phosphorylated Akt rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-GAPDH mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-phosphorylated AMPK mouse polyclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-PGC-1α mouse monoclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-phosphorylated cAMP-response element binding protein (CREB) mouse monoclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-β-Actin mouse polyclonal antibody (1:1,000, Cell Signaling Biotechnology). For the secondary antibody, horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies (1:1,000, GeneTex Inc., Irvine, CA, USA) were used. The blotting proteins were detected by using We-star ECL substrates (Cyanagen, Bologna, Italy). Detected band intensity was analyzed using Chemidoc (Bio-Rad, Hercules, CA, USA).
4. Immunofluorescence staining
Flexor pollicis longus muscle tissues were embedded and frozen at 20°C. Transverse (10 μm thick) were cut on a cryostat and mounted on positively charged slides (Fisher Scientific, Pittsburgh, PA, USA). For immunofluorescence staining sections were fixed with 4% paraformal-dehyde and 4% sucrose in PBS at room temperature for 40 minutes, per-meabilized with 0.5% Nonidet P-40 in PBS, and blocked with 2.5% horse serum and 2.5% bovine serum albumin for 4 hours at room temperature. The sections were incubated with anti-neurofilament-200 (NF200) rabbit polyclonal antibody (1:700, Sigma-Aldrich, St. Louis, MO, USA). Then they were incubated with rhodamine-goat anti-rabbit secondary antibody (1:400, Molecular Probes, Eugene, OR, USA) and Hoechst (1:500, Sigma-Aldrich) for 1 hour at room temperature, and cover-slipped with gelatin mount medium. In cases when the nonspecific sig-nals were high, which occurred in 1-2 cases out of 10 independent ex-periments. The stained samples were viewed with a fluorescence micro-scope (Nikon model E-600, Nikon, Kawasaki, Japan), and the images were captured with a digital camera, and analyzed using Adobe Photoshop Software (version CS6, San Jose, CA, USA).
5. Statistical analysis
All the data is presented as a mean±standard error. Statistical analysis was performed using one-way ANOVA followed by Duncan post hoc test. The significance level was set at p <.05. All graphs were drawn using Prism 6 (GraphPad, La Jolla, CA, USA).
RESULTS
1. Regular exercise decreased body weight after intervention exercise according to exercise sequence and circadian rhythms
Body weight has been known as a to diagnosing obesity index in the rat and they are closely related to the occurrence of various diseases. To determine the difference in body weight according to the exercise sequence and circadian rhythms exercise intervention, we analyzed body weight after exercise intervention. As shown in Fig. 1A, body weight was significantly decreased in the ARMG, RAMG, AREG, and RAEG groups compared to the OCG group, suggesting that exercise application according to regardless of the exercise sequence and circadian rhythms after exercise intervention may be positive effects for improve obesity.
Fig. 1.
Fig. 1.Body weight of obesity rat at the baseline and post. ns, no signifi-cant, ** p<.01 vs the OCG. OCG (n=6), the obesity control group; ARMG (n=6), aerobic-resistance exercise in the morning group; RAMG (n=6), resistance-aerobic exercise in the morning group; AREG (n=6), aerobic-resistance exercise in the evening group; RAEG (n=6), resistance-aerobic ex-ercise in the evening group. 
2. Aerobic-resistance exercise in the evening increased skeletal muscle fiber cross section area
Obesity and muscle size were closely related to muscle torque and power [ 19]. To examine the effect of exercise sequence and circadian rhythms on Type II skeletal muscle fiber cross section area in obese rat after exercise intervention. As shown in Fig. 2A and B, Type II skeletal muscle fiber cross section area was significantly increased in the AREG groups compared to the OCG, ARMG, RAMG, RAEG group after intervention, suggesting that resistance exercise after aerobic exercise in the evening may increase Type II muscle fiber.
Fig. 2.
Fig. 2.Change of CSA in the flexor pollicis longus muscle at post intervention, immunofluorescence staining. A. Flexor pollicis longus muscle immunofluorescence staining images. B. Quantitative analysis on myofiber cross section area. a, p<.001 vs OCG; b, p<.001 vs ARMG; c, p<.001 vs RAMG; e, p<.001 vs RAEG; OCG (n=6), the obesity control group; ARMG (n=6), aer-obic-resistance exercise in the morning group; RAMG (n=6), resistance-aerobic exercise in the morning group; AREG (n=6), aerobic-resistance ex-ercise in the evening group; RAEG (n=6), resistance-aerobic exercise in the evening group. 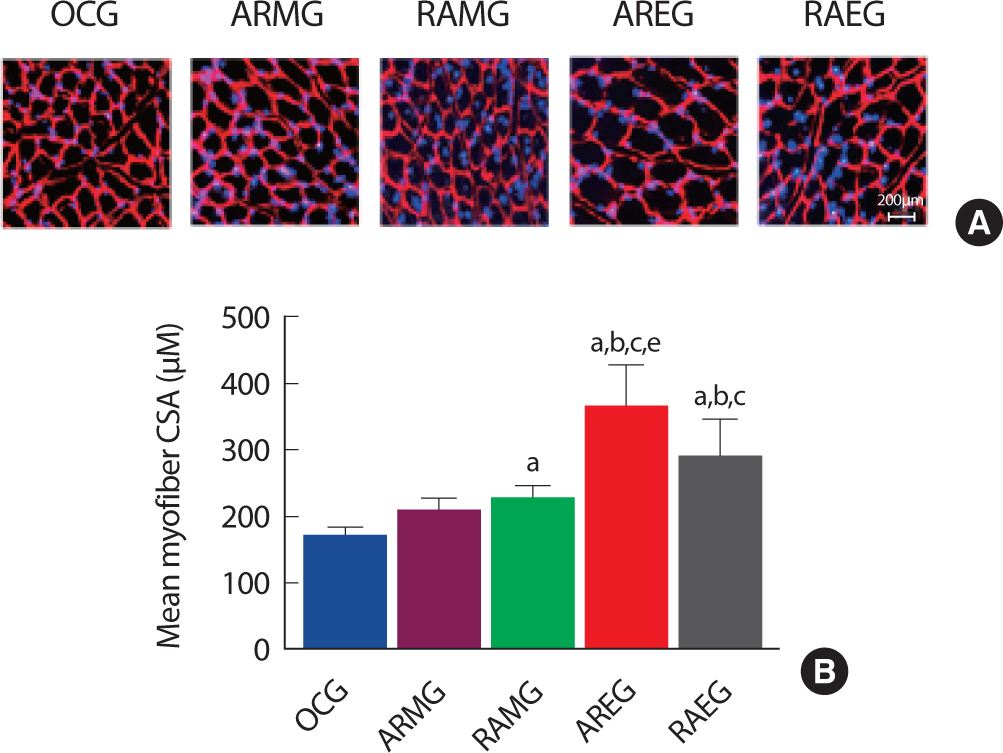
3. Changes of muscle hypertrophy signaling pathway after intervention exercise according to exercise sequence and circadian rhythms
Aerobic and anaerobic exercise have been known as participating in regulating the muscle hypertrophy signaling pathway. To determine the effect of circadian rhythms and exercise sequence on muscle hypertrophy signaling pathway in obese rat after exercise intervention. we analyzed IGF-1, PI3K, p-Akt, p-mTOR at the flexor pollicis longus muscle. As shown in Fig. 3A and B, AREG group was significantly upregulated in IGF-1 expression level compared to the OCG, ARMG, RAMG groups. PI3K was also significantly increased in AREG and RAEG groups than the OCG, ARMG, and RAMG group. p-Akt and p-mTOR were significantly upregulated in AREG compared to all groups after intervention, suggesting that intra-session exercise sequence (aerobic to resistance exercise) in the evening may increase muscle hypertrophy.
Fig. 3.
Fig. 3.The effects of intervention exercise according to exercise sequence and circadian rhythms on muscle hypertrophy related proteins in the flexor pollicis longus muscle. A. Representative western blot images of IGF-1, PI3K, p-Akt, p-mTOR and GAPDH. B. Quantitative analysis on IGF-1, PI3K, p-AKT and p-mTOR/GAPDH ratio. a, p<.05 vs OCG; b, p<.05 vs ARMG; c, p<.05 vs RAMG; e, p<.05 vs RAEG; OCG (n=6), the obesity control group; ARMG (n=6), aero-bic-resistance exercise in the morning group; RAMG (n=6), resistance-aerobic exercise in the morning group; AREG (n=6), aerobic-resistance exercise in the evening group; RAEG (n=6), resistance-aerobic exercise in the evening group. 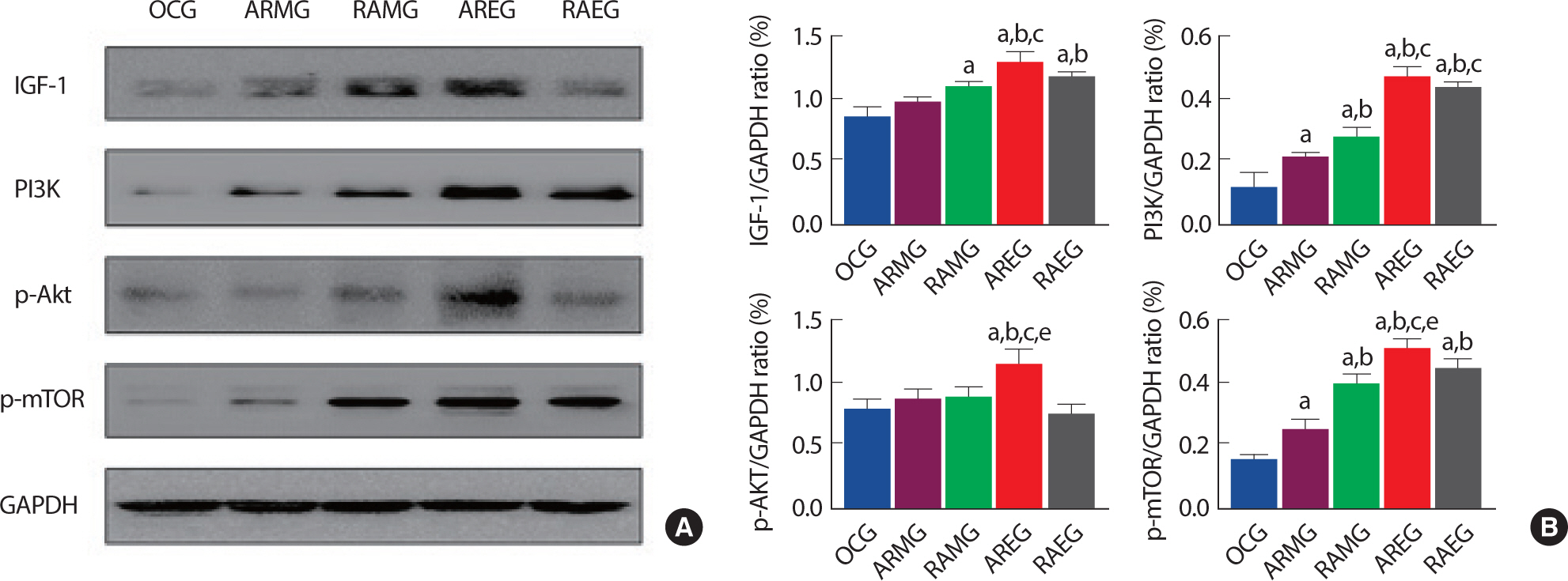
4. Changes of mitochondrial biogenesis signaling pathway after intervention exercise according to exercise sequence and circadian rhythms
Mitochondrial dysfunctions such as low number and activity have been suggested as specific factors in obesity, type 2 diabetes, and metabolic syndrome [ 20]. To confirm changes in mitochondrial biogenesis related proteins expression after intervention, we analyzed p-AMPK, CaMK, PGC-1α, p-CREB at the soleus muscle. As shown in Fig. 4A and B, RAMG group was significantly upregulated in p-AMPK and CaMK expression levels compared to all groups. PGC-1α was also significantly increased in RAMG and AREG groups than the OCG, ARMG, and RAEG groups. RAMG and AREG groups were significantly upregulated in p-CREB expression levels compared to the OCG, ARMG, and RAEG groups after intervention, suggesting that intra-session exercise sequence (resistance to aerobic exercise) in the morning may increase mitochondrial biogenesis.
Fig. 4.
Fig. 4.The effects of intervention exercise according to exercise sequence and circadian rhythms on mitochondrial biogenesis related proteins in the soleus muscle. A. Representative western blot images of p-AMPK, CaMK, p-CREB, PGC-1a and GAPDH. B. Quantitative analysis on p-AMPK, CaMK, p-CREB, PGC-1a/GAPDH ratio (B). a, p<.05 vs OCG; b, p<.05 vs ARMG; d, p<.05 vs AREG; e, p<.05 vs RAEG; OCG (n=6), the obesity control group; ARMG (n=6), aerobic-resistance exercise in the morning group; RAMG (n=6), resistance-aerobic exercise in the morning group; AREG (n=6), aerobic-resistance exercise in the evening group; RAEG (n=6), resistance-aerobic exercise in the evening group. 
DISCUSSION
Obesity not only causes problems in the skeletal muscle metabolic system but is also closely related to disturbance of the circadian clock. The light activated suprachiasmatic nucleus (SCN) of the hypothalamus influences peripheral clock regulation in each organ and explains the relationship between circadian rhythms and obesity [ 21]. The most economical way to prevent obesity is exercise, and circadian rhythms and exercise sequence could be a useful therapeutic tool against obesity. Therefore, in this study was confirmed the weight change according to the circadian rhythms and exercise sequence in rats with obesity. As a results, the body weight was significantly decreased all exercise groups than OCG after intervention. Mohebbi & Azizi [ 22] showed that endurance exercise in the evening significantly reduced weight, while Alizadeh et al. [ 23] demonstrated that morning aerobic exercise was effective in weight loss. However, the mechanism of weight loss according to exercise sequence is still unclear, and research is lacking. Nonetheless, exercise is promoting fat oxidation by increasing mitochondrial biogenesis and function, which is an indirect cause of weight loss. Therefore, regardless of the circadian rhythm and exercise order, exercise seems to be effective in preventing and controlling obesity by reducing body weight. Exercise and circadian rhythms promote muscle hypertrophy through the IGF-1, PI3K, Akt, and mTOR signaling pathways [ 24, 25]. Therefore, our study investigated cross-sectional area and, muscle hypertrophy signaling pathway in the flexor pollicis longus muscle. CSA of the flexor pollicis longus muscle was significantly increased in the AREG compared to OCG, ARMG, RAMG, RAEG. In addition, muscle hypertrophy signaling pathway proteins (IGF-1, PI3K, Akt, ERK1/2, and mTOR) expression levels were the highest in the AREG compared to all groups. Previous study reported that resistance training was more increased the fast twitch fiber (type II) CSA and the muscle hypertrophy index and the width of myotubes of the C2C12 cell line in the evening than morning [ 26, 27]. Intra-session exercise order effect study reported that AMPK activated by aerobic exercise after resistance exercise inhibited mTOR and suppressed muscle hypertrophy [ 28, 29]. The results of previous studies support our findings that endurance-resistance exercise order was performed in the evening might effectively increase fast twitch fiber (type II) CSA and muscle hypertrophy related proteins in obese rat. The main reason for these results is that the sequence of resistance exercise after aerobic exercise is not affected by the interference effect between AMPK and mTOR, as well as muscle hypertrophy is also thought to be caused by the circadian clock markers CLOCK and BMAL1 [ 6, 28, 30- 32]. However, since this study did not identify circadian rhythm regulators at the in vitro, it is necessary to further examine the relationship between circadian rhythm and exercise through future studies. The expression of PGC-1α, a key regulator of energy metabolism and mitochondrial biogenesis, might be regulated by exercise and circadian rhythms. Mitochondrial biogenesis related proteins including p-AMPK and CaMK were the highest in the RAMG compared to other groups. A previous study showed that morning aerobic exercise increased mitochondrial biogenesis and glucose and fatty acid oxidation capacity via the AMPK, p38MAPK, and PGC-1α signaling pathways. Also, aerobic exercise and anaerobic exercise were increased CaMK expression levels regardless of the exercise order [ 33, 34]. The results of previous studies support our findings that p-AMPK and CaMK was increased in the RAMG. In this study found that PGC-1α and p-CREB were upregulated in RAMG and AREG than other groups. A previous study reported that exhaustion exercise test with different exercise sequence was increased PGC-1α expression level regardless of the exercise sequence [ 35, 36]. In addition, De Goede et al. [ 37] reported that mitochondrial respiration and biogenesis were effects of circadian rhythms, exercise, food intake. Circadian rhythms were clearly related to mitochondrial respiration and biogenesis. However, it still controversial in effects of exercise timing to influenced mitochondrial biogenesis. Therefore, additional studies that can further clarify the control of regulators that can affect circadian rhythm are needed.
CONCLUSIONS
We investigated whether the sequence of endurance and resistance training sequence and circadian rhythms could regulate body weight, muscle hypertrophy and mitochondrial biogenesis related molecules. Our findings provide new evidence that muscle CSA and muscle hypertrophy related molecules might be upregulated by resistance exercise after endurance exercise in the evening. In addition, resistance exercise in the morning followed by aerobic exercise might promote fat metabolism and mitochondrial biogenesis.
REFERENCES
1. Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22(1):355-75.   2. Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc. 2003;62(3):621-34.   3. Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. 2006.   4. Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37:737-63.  5. Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38(11):1950-7.   7. Ogasawara R, Sato K, Matsutani K, Nakazato K, Fujita S. The order of concurrent endurance and resistance exercise modifies mTOR signaling and protein synthesis in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2014;306(10):E1155-E62.   8. Han H, Dou J, Hou Q, Wang H. Role of circadian rhythm and impact of circadian rhythm disturbance on the metabolism and disease. J Cardiovasc Pharmacol. 2022;79(3):254-63.   10. Engin A. Circadian rhythms in diet-induced obesity. Adv Exp Med Biol. 2017;19-52.   12. Kim HK, Konishi M, Takahashi M, Tabata H, Endo N, et al. Effects of acute endurance exercise performed in the morning and evening on inflammatory cytokine and metabolic hormone responses. PLoS One. 2015;10(9):e0137567.  13. Küüsmaa M, Schumann M, Sedliak M, Kraemer WJ, Newton RU, et al. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metab. 2016;41(12):1285-94.   14. Kim HK, Ando K, Tabata H, Konishi M, Takahashi M, et al. Effects of different intensities of endurance exercise in morning and evening on the lipid metabolism response. J Sports Sci Med. 2016;15(3):467.   16. Zhang YJ, Li J, Huang W, Mo GY, Wang LH, et al. Effect of electroacu-puncture combined with treadmill exercise on body weight and expression of PGC-1α, Irisin and AMPK in skeletal muscle of diet-induced obesity rats. Zhen Ci Yan Jiu. 2019;44(7):476-80.  18. Kraemer WJ, Flanagan SD, Volek JS, Nindl BC, Vingren JL, et al. Resistance exercise induces region-specific adaptations in anterior pitu-itary gland structure and function in rats. J Appl Physiol. 2013;115(11):1641-7.   19. Abdelmoula A, Martin V, Bouchant A, Walrand S, Lavet C, et al. Knee extension strength in obese and nonobese male adolescents. Appl Physiol Nutr Metab. 2012;37(2):269-75.   21. Froy O. Metabolism and circadian rhythms-implications for obesity. Endocr Rev. 2010;31(1):1-24.   22. Mohebbi H, Azizi M. Maximal fat oxidation at the different exercise intensity in obese and normal weight men in the morning and evening. J Sport Exerc Psychol. 2011;6(1):49-58.  25. Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38(11):1950-7.   26. Sedliak M, Zeman M, Buzgó G, Cvečka J, Hamar D, et al. Effects of time of day on resistance exercise-induced anabolic signaling in skeletal muscle. Biol Rhythm Res. 2013;44(5):756-70.  28. Ezagouri S, Zwighaft Z, Sobel J, Baillieul S, Doutreleau S, et al. Physio-logical and molecular dissection of daily variance in exercise capacity. Cell Metab. 2019;30(1):78-91e74.   29. Perez-Schindler J, Hamilton DL, Moore DR, Baar K, Philp A. Nutritional strategies to support concurrent training. Eur J Sport Sci. 2015;15(1):41-52.   30. Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, et al. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576(2):613-24.  31. Frøsig C, Jørgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5ʼ-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286(3):E411-E417.  32. Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, et al. 5ʼ-AMP-activated protein kinase activity and subunit expression in ex-ercise-trained human skeletal muscle. Journal of J Appl Physiol. 2003;94(2):631-41. 
|
|









