Lee, Lee, and Ha: Cognitive Improvement through Breathing Exercises in Post-Stroke Respiratory Sarcopenia: A Review
Abstract
PURPOSE
The first purpose of this study is to investigate the relationship between Post-Stroke Respiratory Sarcopenia (PSRS) and Cognitive Impairment (CI) through Breathing Exercise (BE) as a respiratory rehabilitation after stroke (ST). The second purpose is to introduce a pilot study design set to investigate and compare the acute effects of each BE to develop a BE protocol for further studies.
METHODS
Pubmed, Scopus, Web of Science and Google Scholar search engines were used to identify the definition and mechanism of ST, CI and respiratory sarcopenia (RS), and to find cases of application of BE in such conditions.
RESULTS
Review; BEs that improve ST, RS, and CI symptoms are Box Breathing (Tactical Breathing), Fast-Breathing, Slow-paced Breathing, Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise. However, the effect is still unclear as post-stroke patients undergo multiple medical treatments other than BE. Pilot Study Prospective results; Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise, Slow-paced Breathing, Box Breathing (Tactical Breathing) and Fast-Breathing will be performed by 40 healthy college students through a randomized controlled trial for 4 weeks. Respiratory functions, exercise intensity, active oxygen level, blood lactate level, cerebral oxygen saturation and cognitive function will be measured pre- and post-intervention along with acute and 2 week mid-intervention. BEs are expected to improve respiratory function, cognitive performance and energy levels while reducing HR, BP, and stress. However, individual response to BE may vary according to health, physical fitness and life styles. All BE will be conducted in an evenly controlled and supervised environment for accurate data collection.
CONCLUSIONS
Further study will be done to develop an appropriate BE protocol for PSRS patients per this review. Follow-up studies may also use this review as a reference for the application of BEs in PSRS patients with CI.
Keywords: Stroke (ST), Respiratory sarcopenia (RS), Cognitive impairment (CI), Breathing exercise (BE), Post-stroke respiratory sarcopenia (PSRS)
INTRODUCTION
Stroke (ST), colloquially known as a brain attack, transpires when there is an interruption in blood supply to the brain [ 1]. The principal mechanism triggering the onset of a ST is either an occluded or ruptured artery [ 2]. An ischemic ST arises when the artery experiences blockage owing to various factors affecting the blood vessels linked to the brain. Conversely, a hemorrhagic ST is induced by elevated intracranial pressure and neuro-nal damage resultant from an arterial rupture within the cerebrum. Cognitive impairment (CI), a condition characterized by difficulties in memory retention, acquisition of new knowledge, focus, or decision-making that impacts daily life, is widespread among the elderly and individuals who have encountered diverse health challenges [ 3]. The etiol-ogy of CI encompasses a range of factors including aging, vascular is-sues, and stroke [ 4- 6]. Such afflictions contribute to an increased mortality rate and a compromised quality of life for patients [ 7, 8]. Consequently, individuals grappling with CI necessitate heightened attention and care. Respiratory Sarcopenia (RS) is a condition typified by the atrophy of muscle fibers and concomitant functional loss within the respiratory muscles, consistent with systemic skeletal muscle deterioration. This multifactorial phenomenon results in both a diminution of respiratory muscle strength and a reduction in muscle mass [ 9]. The anatomical composition of respiratory muscles is vividly delineated in ( Fig. 1) [ 10]. It is pivotal to distinguish RS from respiratory dysfunction; the latter arises from limitations in the ventilatory apparatus or the presence of obstructive pulmonary diseases [ 11]. Additionally, patients suffering from whole-body sarcopenia manifest declines in diaphragmatic muscle thickness in conjunction with decrements in respiratory functionality [ 12]. In line with these obser-vations, RS contributes to impairments in respiratory functions, culmi-nating in diminished oxygen transport and availability to cerebral neurons, thus rendering them susceptible [ 13]. This is of paramount importance, given that the brain accounts for approximately 20% of the body's total oxygen consumption [ 14]. Further accentuating the urgency for increased attention, the formal categorization of sarcopenia as a muscular disease, as endorsed by its ICD-10-CM diagnostic code, underscores its significance [ 15].
Fig. 1.
Fig. 1.Anatomical configuration of respiratory muscles. This figure delineates the anatomical structure of the muscles involved in the inspiratory and expiratory phases of respiration. The inspiratory musculature encompasses the Sternomastoid, Scalenes, External Intercostals, and the Diaphragm. Conversely, the expiratory musculature comprises the Internal Intercostals, External and Internal Obliques, and the Rectus and Transversus abdominis. 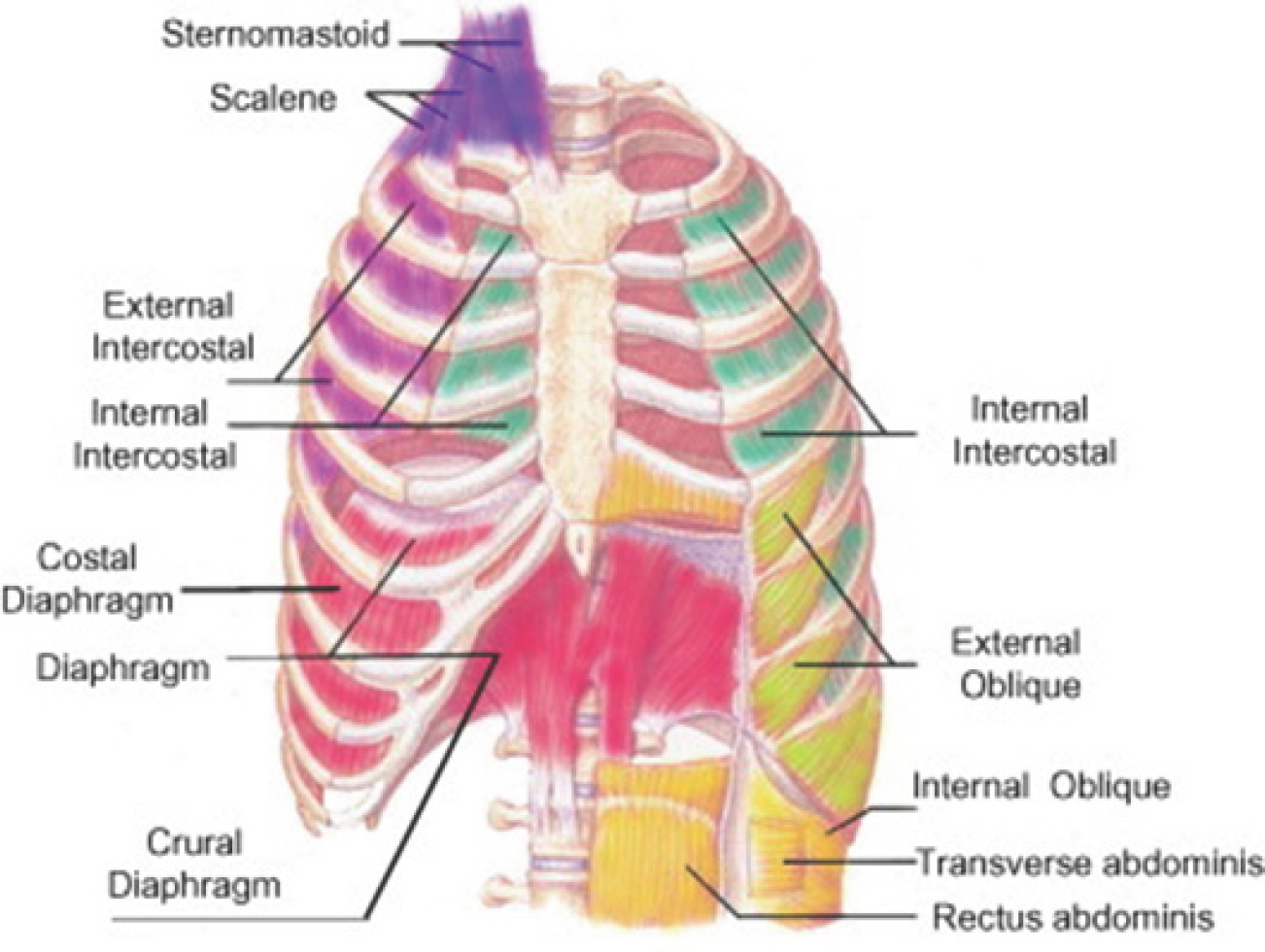
We have delineated Post-Stroke Respiratory Sarcopenia (PSRS) as a de-cline in the strength and mass of the respiratory muscles in individuals who have endured a stroke. Previous study suggests that short time after going through stroke, a decrease in the number of motor unit occurs. This is a consequence of the trans-synaptic inhibition of the spinal alpha motor neurons of the particular muscle [ 16]. Such mechanism explains sarcopenia in skeletal muscles. However, this type of muscle mass and function loss not only occurs in skeletal muscles, but also occurs with high relationship with respiratory muscles [ 17]. Furthermore, stroke in-duces hemiplegia, which causes a decrease in the capability of motor activity in the affected part of the body which leads to decreased physical activity [ 18, 19]. As presented, RS can be triggered by a multitude of health-related adversities. Notably, stroke-induced RS is of critical concern to patients due to the potential proliferation of multifarious side effects, including CI. RS leads to impairments in respiratory functions which leads to decreases in cerebral oxygen inflow and cerebral oxygen saturation. This leads to dysfunction of the prefrontal cortex and eventually affecting especially the executive function, memory, and concentration of individuals [ 20- 22]. However, there exists a paucity of research focusing on stroke-induced RS, thereby accentuating the imperative for a compre-hensive examination in this domain. Breathing exercise (BE), constituted of a series of systematized breathing techniques, is extensively practiced across various fields. It has been documented to ameliorate cardiorespiratory and ventilatory functions, overall quality of life, and cognitive faculties [ 23- 25]. Research indicates that regular physical activity can notably augment the quality of life for individuals across all intensity levels-high, moderate, and even mild [ 26]. ST survivors are susceptible to physical debilitation [ 27], which can im-pede their ability to engage in physical activities and exercises. Hence, the selection of an appropriate exercise intensity is a crucial consideration for stroke patients. BE, performed in a stationary posture, offers an accessible mode of exercise for stroke patients compared to other forms of physical activities. Consequently, the primary objective is to undertake research on BEs as a form of respiratory rehabilitation, which could potentially catalyze cognitive enhancement in individuals afflicted with PSRS based on the interrelationship between ST, RS, and CI, which we scrutinized above. The secondary objective of this study is to present a preliminary study design configured to explore and juxtapose the acute ramifications of different BE modalities. This will serve as the foundation for the formu-lation of a novel BE protocol, positioned to enlighten and direct subse-quent investigations.
METHODS
For this review, the search engines Pubmed ( https://pubmed.ncbi.nlm.nih.gov/), Scopus ( https://www.scopus.com/), Web of Science ( https://www.we-bofscience.com/), and Google Scholar ( https://scholar.google.com/) were uti-lized. The keywords employed for this study included “ Stroke”, “ Sarcopenia”, “ Respiratory Sarcopenia”, “ Cognitive Impairment”, “ Cognitive Function”, “ Respiratory Function”, “ Breathing Exercise”, “ Inspiratory”, “ Expiratory”, “ Diaphragm”, “ Muscle Loss”, “ Muscle Strength”, “ Executive Function”, “ Stroop Task”, “ Pulmonary Function”, “ Brain”, “ Cerebral”, “ Oxy-gen”, “ Respiratory Muscle”, “ Respiratory Strength”, “ Feedback”, “ Biofeedback”, “ IMT”, “ IMT Threshold”, “ POWERbreathe”, “ Breathing”, “ Fast Breathing”, “ Slow Breathing”, “ Inspiratory Diaphragmatic Breathing”, “ Expiratory Pursed-lip Breathing”, “ Box Breathing”, and “ Tactical Breathing”. Previous studies were analyzed to ascertain the definitions and mechanisms underlying ST, CI, and RS, and to identify instances of BE application in such contexts. Additionally, past research was leveraged to discern the relationship between ST, RS, and CI.
RESULTS
1. Breathing Exercise (BE) for Stroke (ST)
BE for ST was composed of Inspiratory Diaphragmatic Breathing+ Expiratory Pursed-lip Breathing Exercise, Slow-paced Breathing Exercise and Fast-Breathing Exercise. Inspiratory Diaphragmatic Breathing+ Expiratory Pursed-lip Breathing Exercise for ST patients showed significant improvements in respiratory functions such as FVC, FEV1, FVC/FEV1 ratio, PEF, TV, IC, and physical activity levels including chest expansion and 6-minute walk test outcomes. Furthermore, it improved activation of respiratory muscles in the UT, LD, RA, and IAO. Slow-paced Breathing Exercise for ST showed increase in BRS, up-sequence of the BRS and HRV, as well as decrease in SBP and HR. Fast-Breathing Exercise showed significant decrease in SBP and DBP. These effects of BEs are crucial for ST patients’ outcomes. Precise information is visually delineated in Table 1.
Table 1.
Effects of Breathing Exercise (BE) in Stroke (ST) patients
|
Author |
Year |
BE Type |
Participant characteristics |
Measured variables |
Measurement period |
Protocol |
Results |
|
Seo KC et al. [28] |
2013 |
Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise |
n=30 (M: 17, F: 13) Age (years): 61.5±2.8 Chronic stroke patients |
FVC, FEV1, FEV1/FVC, PEF, TV, VC, IRV, ERV, IC |
Pre- & Post-Intervention |
5 times/week Total 4 weeks 15 minutes/session |
Significant improvements in FVC/FEV1, TV, IC |
|
Seo K et al. [29] |
2017 |
Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise |
n=30 (M: 15, F: 15) Age (years): 63.6±3.7 Stroke Patients |
Electromyographic measurement, UT, LD, RA, EAO, IAO |
Pre- & Post-Intervention |
5 times/week Total 4 weeks 15 minutes/session |
Significant improvements in activation of UT, LD, RA, IAO |
|
Yoon JM et al. [30] |
2022 |
Diaphragmatic Breathing+Pursed Lip Breathing Exercise |
n=32 Older stroke patients |
FVC, FEV1, FEV1/FVC, PEF, Chest expansion, 6-minute walk test |
Pre- & Post-Intervention |
Total 4 weeks |
Significant improvements in chest expansion 6-minutewalk test FVC, FEV1, PEF |
|
Larson M et al. [31] |
2020 |
Slow-paced Breathing |
n=12 (M: 9, F: 3) Age (years): 52±13 Stroke patients |
BP, HR, BRS, BRSup, BRSdown, HRV |
Pre-, during- & Post-Intervention |
Acute 15 minutes |
Increase in BRS, BRSup, HRV decrease in SBP, HR |
|
Mourya M et al. [32] |
2009 |
Fast-Breathing |
n=60 Age (years): 20-60 Stage 1 Hypertension |
Autonomicfunction test, S/L ratio, 30:15 ratios, valsalva ratio, E/I ratio, hand grip test, cold pressor response |
Pre- & Post-Intervention |
15 minutes/session 2 Sessions/day daily Total of 3 months |
Significant decrease in SBP, DBP |
2. Breathing Exercise (BE) for Respiratory Sarcopenia (RS)
BE for RS included Box Breathing (Tactical Breathing) and Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise. Box Breathing (Tactical Breathing) showed significant improvements in respiratory functions which include FVC, FEV1, and FIVC. Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise showed significant improvements in respiratory functions such as FVC, FVC/FEV1, TV, IC, and SVC. In addition, it showed improvements in respiratory muscle activation of the UT, LD, RA, and IAO. Such results support the mechanism of BE being a respiratory rehabilitation program for RS. The specific information is visually outlined in Table 2.
Table 2.
Breathing Exercise (BE) for Respiratory Sarcopenia (RS)
|
Author |
Year |
BE Type |
Participant characteristics |
Measured variables |
Measurement period |
Protocol |
Results |
|
Ahmed A et al. [33] |
2021 |
Box breathing (Tactical breathing) |
n=30 (M: 15, F: 15) Age (years): 18-25 Healthy college students |
FVC, FEV1, FEV1/FVC, FEF 25-75, PEFR, FIVC |
Pre- & Post-Intervention |
2 sessions/day Total of 30 days 20 times/session |
Significant improvements in FVC, FEV1, FIVC |
|
Seo KC et al. [28] |
2013 |
Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise |
n=30 (M: 17, F: 13) Age (years): 61.5±2.8 Chronic stroke patients |
FVC, FEV1, FEV1/FVC, PEF, TV, VC, IRV, ERV, IC |
Pre- & Post-Intervention |
5 times/week Total 4 weeks 15 minutes/session |
Significant improvements in FVC/FEV1, TV, IC |
|
Yong MS et al. [34] |
2017 |
Diaphragmatic breathing exercise |
n=31 Age (years): 21.8±1.61 Healthy participants |
FVC, SVC |
Pre- & Post-Intervention |
Acute |
Significant improvements in FVC, SVC |
|
Seo K et al. [29] |
2017 |
Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing Exercise |
n=30 (M: 15, F: 15) Age (years): 63.6±3.7 Stroke patients |
Electromyographic measurement, UT, LD, RA, EAO, IAO |
Pre- & Post-Intervention |
5 times/week Total 4 weeks 15 minutes/session |
Significant improvements in activation of UT, LD, RA, IAO |
3. Breathing Exercise (BE) for Cognitive Impairment (CI)
BE for CI included Slow-paced Breathing and Tactical Breathing (Box Breathing). Slow-paced Breathing resulted in better performance on executive function tasks and cognitive stress tasks. Tactical Breathing (Box Breathing) resulted in less physiological arousal and stress reduction. Accurate information is distinctly presented in Table 3.
Table 3.
Breathing Exercise (BE) for Cognitive Impairment (CI)
|
Author |
Year |
BE Type |
Participant characteristics |
Measured variables |
Measurement period |
Protocol |
Results |
|
Laborde S et al. [35] |
2021 |
Slow-paced Breathing |
n=78 (M: 41, F: 37)
Age (years): 23.33
Healthy participants with no chronic conditions |
Cardiac vagal activity inhibition task (Stroop task)
Working memory
Capacity (AOSPAN)
Cognitive flexibility (Modified card sort test) |
Cardiac vagal activity (Pre- & Post-Intervention)
Executive function (Post-Intervention) |
3 sets
5 minutes/set
1 minute rest between set
Acute |
Better performance on executive function tasks compared to control group |
|
Laborde S et al. [36] |
2017 |
Slow-paced Breathing |
n=16 (M: 14, F: 2)
Age (years): 17.39
Adolescents with developmental disabilities |
Cognitive stress task (Kaufman-assessment battery of children)
Heart rate variability recording |
Cognitive stress task (Post-intervention)
Heart rate variability
Recording (Pre- & Post-intervention) |
3 sets
5 minutes/set
1 minute rest between set
Acute |
Better performance on cognitive stress task compared to control group |
|
Röttger S et al. [37] |
2021 |
Tactical Breathing (Box Breathing) |
n=30 (M: 24, F: 6)
Age (years): 24.2±2.3
Healthy adults |
Stroop task
Subjective strain (MDMQ)
Breathing rate
Sympathetic activity
HR, HRV
Electrodermal activity |
Subjective strain (Pre- & Post-intervention)
Stroop task (Post-intervention)
Others (Pre-, Mid- & Post-intervention) |
Acute 6 minutes |
Less physiological arousal in Tactical Breathing compared to the other group |
|
Bouchard S et al. [38] |
2012 |
Tactical Breathing (Box Breathing) |
n=41
Age (years): 18-60
Soldiers with basic stress management training & first aid training in combat |
HR, Salivary cortisol |
HR (Baseline, During an apprehension phase, during the live simulation)
Salivary cortisol (Waking up, before and after the live simulation) |
30 minutes/session
1 session/day
Total of 3 days |
Significant stress reduce compared to control group |
4. Non-Equipment-based Breathing Exercise VS. Equipment-based Breathing Exercise
We compared non-equipment-based BEs and equipment-based BEs in order to identify the difference in protocols and results of these two different methods. Resultingly, both methods showed beneficial effects on physiological factors such as respiratory function, as well as physical fitness levels. However, whereas non-equipment-based BE demonstrated positive effects on cognitive function, the impact of equipment-based BE on cognitive function remains inadequately supported by existing studies. Therefore, a systemized non-equipment-based BE protocol can be an effective program for PSRS compared to equipment-based BE. Visual information is supported in Table 4.
Table 4.
Comparison of non-equipment-based breathing exercise and equipment-based breathing exercise
|
Author |
Year |
Use of equipment |
Type |
Participant characteristics |
Measured variables |
Measurement period |
Protocol |
Results |
|
Ahmed A et al. [33] |
2021 |
Non-equipment-based |
Box Breathing (Tactical Breathing) |
n=30 (M: 15, F: 15)
Age (years): 18-25
Healthy college students |
FVC, FEV1, FEV1/FVC, FEF 25-75, PEFR, FIVC |
Pre- & Post-Intervention |
2 sessions/day Total of 30 days 20 times/session |
Significant Improvements in FVC, FEV1, FIVC |
|
Laborde S et al. [35] |
2021 |
Non-equipment-based |
Slow-paced Breathing |
n=78 (M: 41, F: 37)
Age (years): 23.33
Healthy participants withno chronic conditions |
Cardiac vagal activity inhibition task (Stroop task), Working memory
Capacity(AOSPAN), Cognitive flexibility (Modified card sort test) |
Cardiac vagal activity (Pre- & Post-Intervention)
Executive function (Post-Intervention) |
3 sets
5 minutes/set
1 minute rest between set
Acute |
Better performance on executive function tasks compared to control group |
|
Yoon JM et al. [30] |
2022 |
Non-equipment-based |
Diaphragmatic
Breathing+Pursed-lip
Breathing Exercise |
n=32 Older stroke patients |
FVC, FEV1, FEV1/FVC, PEF, Chest expansion, 6-minute walk test |
Pre- & Post-Intervention |
Total 4 weeks |
Significant improvements in chest expansion, 6-Minute walk test FVC, FEV1, PEF |
|
Mourya M et al. [32] |
2009 |
Non-equipment-based |
Fast-Breathing |
n=60
Age (years): 20-60
Stage 1 hypertension |
Autonomic function test, S/L ratio, 30:15 ratios, Valsalva ratio, E/I ratio, Hand grip test, Cold pressor response |
Pre- & Post-Intervention |
15 minutes/session
2 sessions/day daily
Total of 3 months |
Significant decrease in SBP, DBP |
|
da Silva Guimarães B et al. [39] |
2021 |
Equipment-based |
POWERbreathe K-5 |
n=101 (M: 49, F: 52)
Age (years): 18-86
Tracheostomized patients on prolonged weaning |
Respiratory muscle strength (MIP, Timed inspiratory effort Index), ICU Survival rate, Weaning success |
Pre- & Post-Intervention |
30 breaths/set
2 sets/session
1 session/day
5 days/week |
Improvements in respiratory muscle strength (MIP, Timed inspiratory effort index), ICU survival rate, Weaning success |
|
Kaeotawee P et al. [40] |
2022 |
Equipment-based |
Threshold IMT |
n=60 (M: 42, F: 18)
Age (years): 8-15
Obese children/Adolescents |
FEV1, FEV, FEV1/FVC, FEF25-75, PEF, Respiratory muscle strength (MIP, MEP),6-MWT |
Pre- & Post-Intervention |
30 breaths/session
2 sessions/day
Total of 8 weeks |
Improvements in 6-MWT, MIP |
|
Lee HY et al. [41] |
2014 |
Equipment-based |
Feedback device |
n=22
Age (years): 6-12
Children with cerebral palsy |
Pulmonary function test (FVC, FEV1, PEF, VC, TV, IRV, ERV) |
Pre- & Post-Intervention |
15 minutes/session
3 days/week
Total of 4 weeks |
Improvements in FVC, FEV1 |
5. Summary
ST stands as a significant precursor to CI and RS. We have formulated a mechanism wherein RS functions as a mediator for CI in ST patients. Within this framework, we propose the hypothesis that BE hold the potential to enhance cognitive function or ameliorate CI. We are set to em-bark on a pilot study, and the anticipated acute impacts of the BEs on college students include the following:
1. Inspiratory Diaphragmatic Breathing and Expiratory Pursed-lip Breathing Exercise are projected to bolster respiratory function, allevi-ate anxiety levels, and augment focus and attention. 2. Implementing the Box Breathing Exercise (Tactical Breathing) is pre-dicted to diminish stress and fortify cognitive function. 3. Fast-Breathing Exercise is anticipated to amplify alertness and boost energy levels, whereas. 4. Slow-paced Breathing Exercise is foreseen to decrease heart rate, blood pressure, and stress levels.
Nonetheless, it is crucial to recognize that individual responses to BE may vary, influenced by factors such as health status, physical fitness, physical capacity, and lifestyle. To ensure the accuracy of data collection, all BE sessions will be conducted in a rigorously controlled and supervised environment. Our hypothesis is visually delineated in Fig. 2.
Fig. 2.
Fig. 2.Implementation of BE in the interplay between ST, RS, and CI. ST culminates in CI, with RS serving as an intermediary factor. The impact of imple-menting BE (comprising Inspiratory Diaphragmatic Breathing+Expiratory Pursed-lip Breathing, Box Breathing/Tactical Breathing, Slow-paced Breathing, and Fast-Breathing) will be ascertained through the course of our investigation. 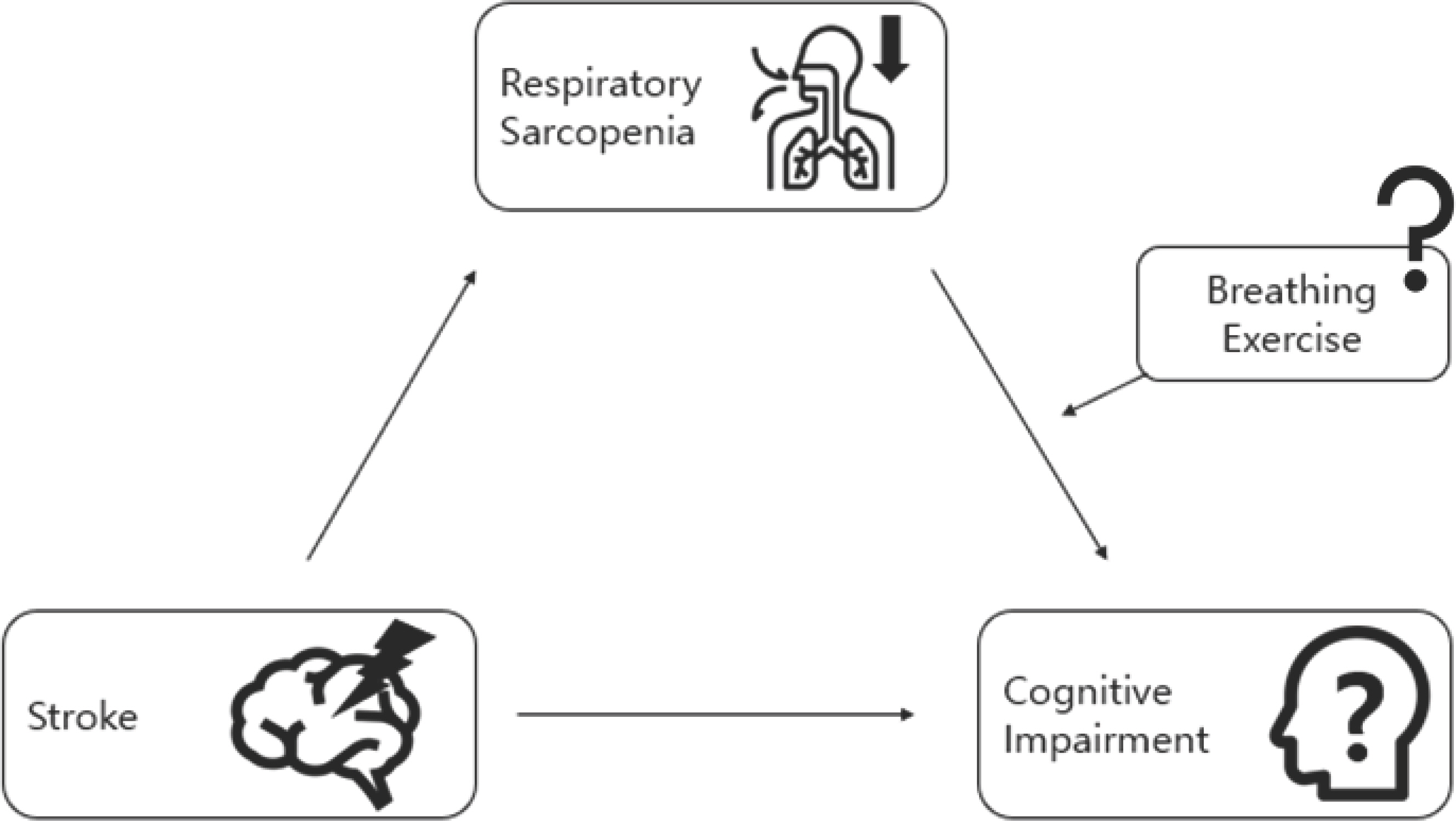
DISCUSSIONS
We suggest a pilot study aimed at substantiating the acute and short-term effects of Inspiratory Diaphragmatic Breathing Exercise+Expiratory Pursed-lip Breathing Exercise, Slow-paced Breathing, Box Breathing (Tactical Breathing) and Fast-Breathing Exercise. Our pilot study will be composed of 3 phases. Phase 1 will be finding the acute effect of each breathing exercises. Phase 2 will be finding the effects of practicing each breathing exercises for 2-week. Phase 3 will be finding the effects of practicing each breathing exercises for 4-week. Previous studies showed a minimum of 2-week breathing exercise protocol and a maximum of 4-week breathing exercise protocol. Therefore, the purpose of our pilot study is to find the most efficient and effective breathing exercise protocol. Furthermore, each breathing exercise will be executed 5 days a week, while performing the exercise for 15 minutes per day. Follow-up study will be executed to determine the combination effects of each BE. Insights obtained from this phase will then be used to develop a new type of BE.
Sample size for this study was calculated using G-power, version 3.1. A power analysis with a desired effect size of 0.25 (default), significance level of 0.05, and power of 0.80 determined that a sample size of 36 was required. 40 people will be recruited considering the drop-outs, and the recruited individuals will be randomly distributed into 4 breathing exercise groups. There will be a total of 4 measurement periods throughout the study. This study will be conducted in University of Seoul, Seoul, Republic of Korea on 40 healthy college students. Participants will be recruited through online and offline recruitment advertisements.
Measurement variables will encompass a range of physiological and cognitive parameters. Respiratory functions, comprising Forced Vital Capacity (FVC), Forced Expiratory Volume in 1 second (FEV1), Maximal Inspiratory Pressure (MIP), and Maximal Expiratory Pressure (MEP), will be measured utilizing the Bionet Cardio7 (Bionet America, Inc., USA). Exercise intensity includes Rate of Perceived Exertion (RPE) and Heart Rate (HR), which will be measured pre-, mid-, and post-intervention, utilizing the Polar monitoring system (Polar Electro, Finland). Active oxygen levels will be assessed through the BioDoctor BS-502 device (Bionics, Republic of Korea), while blood lactate levels will be ascertained utilizing the Accutrend Plus Analyzer (Roche Diagnostics, Switzerland). Pre- and post-intervention measurements of cerebral oxygen saturation will be measured during the administration of the Neurocognitive test CNS Vital Signs, along with the fNIRS device (OBELAB, Korea). Cognitive function, germane to the CNS Vital Signs, will be evaluated as well. All variables will undergo pre- and post-intervention measurements, while additional measurement of exercise intensity mid-intervention will be conducted. Our pilot study is visually presented in Fig. 3.
Fig. 3.
Fig. 3.Pilot study design. A randomized controlled trial of 4 interventions and 4 measurements will be conducted. A, Pre-Intervention measurement; B, Measurement after acute Breathing Exercise; C, Measurement after 2-week Breathing Exercise; D, Measurement after 4-week Breathing Exercise. 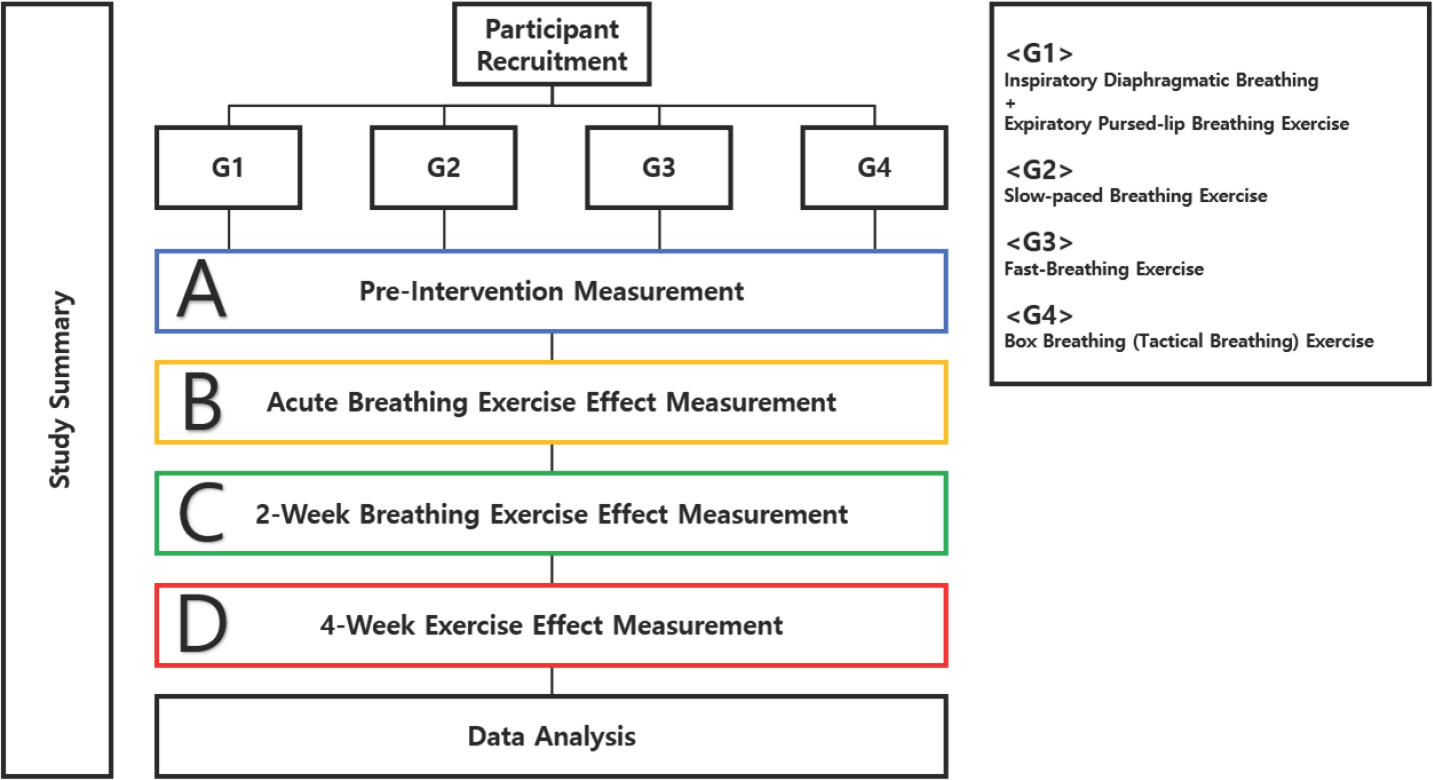
Results of this current review have shown the effects of breathing exercises on ST, RS, and CI. However, previous studies have been conducted in participants with various characteristics which makes it difficult to consider a superior BE. Furthermore, there was a lack of studies particu-larly targeting PSRS patients. Therefore, there is a need of further study on investigating the effects of BE on PSRS patients. PSRS patients are high risk groups which makes is crucial to verify the effectiveness and safety of BE before application.
CONCLUSION
CI followed by respiratory muscle mass and function loss in ST patients are critical. In addition, the improvement of respiratory function, cognitive performance, and physiological parameters through BE in various cases has been confirmed. Still there is a need of development of standardized BE protocols for PSRS patients. Therefore, further study will be conducted to establish an appropriate BE protocol for PSRS patients based on this review. The results of our pilot study will be used to develop a new BE protocol which will undergo validation through application to college students in order to affirm its safety and feasibility. After verifying the safeness of the new BE protocol, we will gradually apply it to healthy older adults, ST patients, and PSRS patients. Future follow-up studies may also use this paper as a reference, orienting the application of BE in PSRS patients with CI.
REFERENCES
2. Shiber JR, Fontane E, Adewale A. Stroke registry: hemorrhagic vs ischemic strokes. Am J Emerg Med. 2010;28(3):331-3.   5. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-17.   6. Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc. 2002;50(4):700-6.   7. Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. Bmj. 1996;312(7031):608-11.  8. Hill NL, McDermott C, Mogle J, Munoz E, DePasquale N, et al. Subjective cognitive impairment and quality of life: a systematic review. Int Psychogeriatr. 2017;29(12):1965-77.  10. Ratnovsky A, Elad D, Halpern P. Mechanics of respiratory muscles. Respir Physiol Neurobiol. 2008;163(1-3):82-9.   14. Vinokurov AY, Stelmashuk OA, Ukolova PA, Zherebtsov EA, Abramov AY. Brain region specificity in reactive oxygen species production and maintenance of redox balance. Free Radic Biol Med. 2021;174:195-201.   16. Arasaki K, Igarashi O, Ichikawa Y, Machida T, Shirozu I, et al. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci. 2006;250(1-2):27-32.   18. Yoon J, Park J, Lee D, Roh H. Comparisons of respiratory function and activities of daily living between spinal cord injury and stroke patients and normal elderly people. J Phys Ther Sci. 2012;24(6):465-9.  19. Van der Slot WM, Roebroeck ME, Landkroon AP, Terburg M, Van den Berg-Emons RJ, et al. Everyday physical activity and community participation of adults with hemiplegic cerebral palsy. Disabil Rehabil. 2007;29(3):179-89.   20. Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14(3):329-40.   23. Dhungel KU, Malhotra V, Sarkar D, Prajapati R. Effect of alternate nostril breathing exercise on cardiorespiratory functions. Nepal Med Coll J. 2008;10(1):25-7.  25. Kang ES, Yook JS, Ha MS. Breathing exercises for improving cognitive function in patients with stroke. J Clin Med. 2022;11(10):2888.  26. Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45(5):649-57.
27. Heshmatollah A, Mutlu U, Koudstaal PJ, Ikram MA, Ikram MK. Cognitive and physical impairment and the risk of stroke-A prospective cohort study. Sci Rep. 2020;10(1):6274.   28. Seo KC, Lee HM, Kim HA. The effects of combination of inspiratory diaphragm exercise and expiratory pursed-lip breathing exercise on pulmonary functions of stroke patients. J Phys Ther Sci. 2013;25(3):241-4.  30. Yoon JM, Im SC, Kim K. Effects of diaphragmatic breathing and pursed lip breathing exercises on the pulmonary function and walking endurance in patients with chronic stroke: a randomised controlled trial. Int J Ther Rehabil. 2022;29(8):1-11.  32. Mourya M, Mahajan AS, Singh NP, Jain AK. Effect of slow-and fast-breathing exercises on autonomic functions in patients with essential hypertension. J Altern Complement Med. 2009;15(7):711-7.   33. Ahmed A, Devi RG, Priya AJ. Effect of box breathing technique on lung function test. J Pharm Res Int. 2021;33(58A):25-31.   35. Laborde S, Allen MS, Borges U, Hosang TJ, Furley P, et al. The influ-ence of slow-paced breathing on executive function. J Psychophysiol. 2021;36(1):13-27.  39. da Silva Guimarães B, de Souza LC, Cordeiro HF, Regis TL, Leite CA, et al. Inspiratory muscle training with an electronic resistive loading device improves prolonged weaning outcomes in a randomized controlled trial. Crit Care Med. 2021;49(4):589-97.  
|
|








