Association of Characteristics between Acute Stroke Patients and Sarcopenia: A Cross-Sectional Study
Article information
Abstract
PURPOSE
This study aimed to identify the prevalence of sarcopenia among acute stroke patients, differences in characteristics based on the presence of sarcopenia, the association between functional and sarcopenia factors, and the association between characteristics and the presence of sarcopenia.
METHODS
This study was conducted using a cross-sectional design. Sixty-two stroke patients volunteered to participate and were assigned to the sarcopenia group (n=32) and the non-sarcopenia group (n=30). All data collection, including assessment of general characteristics, sarcopenia factors and functional factors, was completed within one day.
RESULTS
A sarcopenia prevalence rate of 51% was observed. As skeletal muscle mass index (SMI) and grip strength increased, the berg balance scale (BBS) also increased. Additionally, as grip strength increased, the modified Barthel index (MBI) increased. Significant differences between groups were observed in the characteristics of age, weight, mini-mental state examination, SMI, grip strength, manual muscle testing, BBS, functional ambulation category, and MBI. Furthermore, with each increase of 1 in SMI, the probability of belonging to the sarcopenia group decreased by 0.204 times.
CONCLUSIONS
A high prevalence of sarcopenia was observed in acute stroke patients, with differences in characteristics between stroke patients without sarcopenia and those with sarcopenia. As sarcopenia factors increased, BBS and MBI also increased. Increasing SMI in acute stroke patients can reduce the risk of sarcopenia diagnosis; therefore, exercise interventions aimed at increasing SMI should be considered.
INTRODUCTION
Sarcopenia is generally defined as the loss of skeletal muscle mass, quality, and strength associated with aging [1]. It has been officially classified as a clinical condition in the 10th revision of the international classification of diseases (ICD-10) in 2016 and the 8th edition of the korean standard classification of diseases (KCD-8) in 2021 [2,3]. This condition impacts the progression of frailty, increases the risk of falls and fractures, ultimately leading to hospitalization and mortality [4-6]. Therefore, it is crucial to prevent sarcopenia or detect it early for intervention.
Stroke is a central nervous system disorder caused by cerebral infarction or intracerebral hemorrhage [7]. It is one of the major causes of disability worldwide [8]. Stroke patients are affected by functional factors such as balance, gait, and activities of daily living due to hemiparesis [9]. Currently, rehabilitation for stroke patients in clinical practice focuses on functional recovery [10].
Sarcopenia is frequently observed in survivors of stroke, with a higher prevalence during the acute phase [11]. Given the close association between sarcopenia and aging, as well as the high incidence of stroke in the elderly population, many stroke patients may already have sarcopenia at the time of stroke onset [12]. While functional recovery-focused rehabilitation is common for stroke patients, resistance exercise aimed at increasing muscle mass and strength is recommended for sarcopenia [13-15]. However, it is necessary to determine which factors should be the focus of rehabilitation for stroke patients with sarcopenia.
There have been previous studies reporting an association between stroke patients and sarcopenia. One previous study found that older age, severe stroke, low BMI, poor swallowing function, and a history of previous stroke during acute hospitalization were associated with sarcopenia among acute stroke patients [16]. Another previous study reported that tube feeding, high stroke severity, decreased non-hemiplegic calf circumference, and older age were associated with sarcopenia among subacute stroke patients [17]. Previous studies have been limited by their failure to include functional factors in stroke patients. Therefore, further studies are needed to determine the association between functional factors and sarcopenia in stroke patients.
This study aimed to identify the prevalence of sarcopenia among acute stroke patients, identify differences in characteristics based on the presence of sarcopenia, identify the association between functional factors and sarcopenia factors, and identify the association between characteristics and the presence of sarcopenia.
METHODS
1. Participants
This study included a total of 62 acute stroke patients admitted to G Rehabilitation Hospital located in Gwangju. The study was conducted with approval from the institutional review board (IRB) of Nambu University (IRB: 1041478-2023-HR-015). Participants voluntarily consented to participate in the study either by themselves or through their guardians, and the hospital granted permission for their participation. The in-clusion criteria for participants were individuals diagnosed with stroke within the past three months, categorized as acute stroke. Exclusion criteria were as follows: individuals with cardiovascular diseases or orthopedic conditions that could pose risks or influence participation in the study, individuals with a level of consciousness below stupor, and individuals unable to perform grip strength evaluation with at least one hand.
2. Experimental procedure
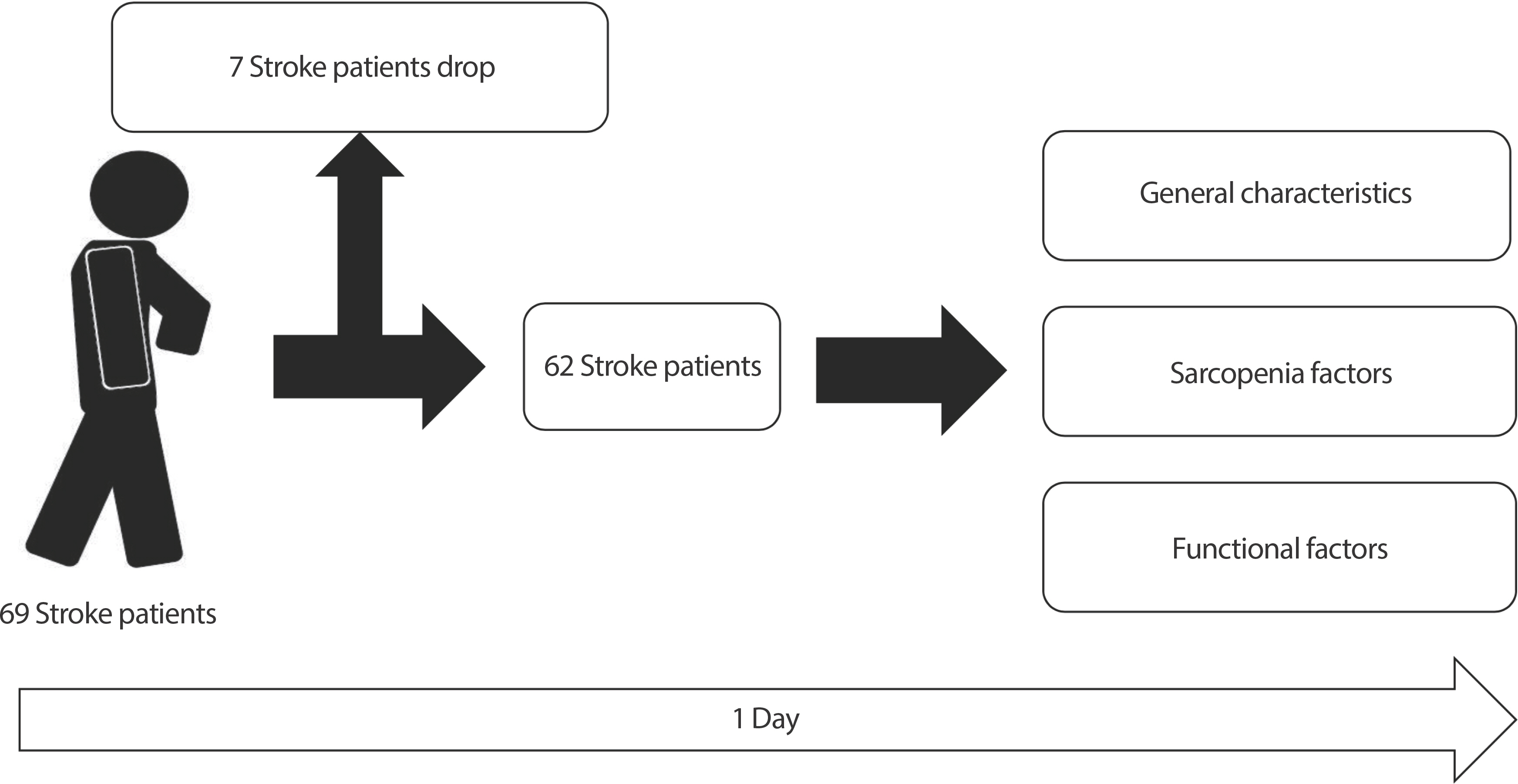
This study was conducted using a cross-sectional design (Fig. 1). Of the 69 stroke patients who participated in the study, 7 were excluded for meeting the exclusion criteria. All data collection, including assessment of general characteristics, sarcopenia factors and functional factors, was completed within one day.
3. General characteristics data collection
General characteristics were collected from the participants latest data in the electronic medical record (EMR). Data on sex, type of stroke, age, height, weight, and mini-mental state examination (MMSE) were obtained from the EMR. The MMSE within the EMR system utilized the most recent available data. Occupational therapists assess the MMSE within three days of stroke patients being admitted to the hospital, with subsequent reassessments occurring on a monthly basis. The collection of EMR data was conducted with approval obtained from the IRB, participants, or their guardians prior to study participation.
4. Sarcopenia factors data collection
The diagnostic criteria for sarcopenia were based on the Asian working group for sarcopenia 2019 criteria [18]. To diagnose sarcopenia, both skeletal muscle mass index (SMI) and grip strength were evaluated. Sarcopenia was diagnosed when both SMI and grip strength were below the criteria. Criteria for SMI are <7.0 kg/m² in men and <5.7 kg/m² in women. The grip strength criteria are <28 kg for men and <18 kg for women. SMI was assessed using the bioelectrical impedance analysis method with BWA2.0 (Inbody, Seoul, Korea). Depending on the participant, s condition, evaluations were conducted in a supine or standing position as recommended by the assessment tool. Grip strength was evaluated using a Jamar hydraulic hand dynamometer (B&L Engineering®, CA, USA). If both hands were measurable, the higher value was used. If only one hand was measurable, the value from that hand was used.
5. Functional factors data collection
1) Manual muscle testing
This study utilized manual muscle testing (MMT) to evaluate participants, muscle strength. MMT assesses twelve upper and lower extremity muscle groups bilaterally, including shoulder flexion, shoulder abduction, elbow flexion, elbow extension, wrist flexion, wrist extension, hip flexion, hip extension, knee flexion, knee extension, dorsiflexion, and plantar flexion. Each muscle group is graded on a scale: Z grade=1 point, T grade=2 points, P grade=3 points, F grade=4 points, G grade=5 points, and N grade=6 points [9]. These scores were then summed to obtain a total MMT score, with a maximum possible score of 144 points.
2) Berg balance scale
The Berg balance scale (BBS) is an assessment tool used to evaluate balance in stroke patients, known for its high reliability and validity [19,20]. The BBS is utilized to objectively determine a participant, s ability to safely balance during a series of predetermined tasks. The researcher assessed 14 items divided into three categories: sitting, standing, and postural changes. Each item is scored on a scale of 0 to 4 points.
3) Functional ambulation category
The functional ambulation category (FAC) is an assessment tool used to evaluate gait in stroke patients, known for its high reliability and validity [21]. The researcher determines the amount of human support the participant requires when walking, regardless of whether they use a per-sonal assistive device. Each item is scored on a scale of 0 to 5 points.
4) Modified bathel index
The modified Barthel index (MBI) is an assessment tool used to evaluate activities of daily living in stroke patients, known for its high reliability and validity [22]. The researcher divided the 10 activities of daily living into detailed items and rated the degree of assistance required for each item on a scale of 0 to 5 points.
6. Statistical analysis
The sample size of 52 participants was calculated using the G*Power program with an effect size of 0.8, power of 0.80, and a significance level of 0.05. Considering an approximate dropout rate of 20%, the final sample size was determined to be 62 participants. All statistical data analyses were performed using the Statistical Package for the Social Sciences SPSS 20.0 software. All data, except for those representing frequencies, were expressed as mean±standard deviation. Independent t-tests were conducted to compare the two groups. Multiple regression analysis was used to check the effect of sarcopenia factors on functional factors. Additionally, hierarchical logistic regression analysis was performed to investigate the significant effects on sarcopenia. The statistical significance level was set at 0.05.
RESULTS
Sixty-two acute stroke patients participated. Among them, thirty-two participants were diagnosed with sarcopenia, resulting in a sarcopenia prevalence rate of 51%. The comparison between the sarcopenia group and the non-sarcopenia group is presented in Table 1. Age, weight, MMSE, SMI, grip strength, MMT, BBS, FAC, and MBI showed significant differences between the groups (p<.05). However, there were no significant differences in sex, type of stroke, and height between the groups (p>.05). The results of the analysis of the effects of sarcopenia factors on functional factors in acute stroke patients are presented in Table 2. As SMI and grip strength increase, BBS also increases. In particular, it is more influenced by SMI than grip strength. Additionally, as grip strength increases, MBI also increases. The results confirming the effect of sarcopenia in this study are presented in Table 3. Only SMI showed significance in all models (p<.05). With each increase of 1 in SMI, the probability of belonging to the sarcopenia group decreased by 0.204 times.
DISCUSSION
This study revealed a sarcopenia prevalence rate of 51%. As SMI and grip strength increase, BBS also increases. Additionally, as grip strength increases, MBI also increases. Significant differences between groups were observed in the characteristics of age, weight, MMSE, SMI, grip strength, MMT, BBS, FAC, and MBI. Specifically, it was found that with each increase of 1 in SMI, the probability of belonging to the sarcopenia group decreased by 0.204 times.
The prevalence of sarcopenia in stroke patients varies according to reported studies. A meta-analysis study investigating the prevalence of sarcopenia in stroke patients reported an integrated prevalence estimate of 42% [11]. The higher prevalence rate found in this study compared to previous research is believed to be due to differences in the onset of stroke among stroke patients included in the study. Other previous studies have reported similar prevalence rates to this study. Results from investigations into the prevalence of sarcopenia among acute stroke patients admitted to rehabilitation wards showed prevalence rates of 48.3% and 53.5% [23,24]. This is attributed to the increased prevalence of sarcopenia during the acute phase of stroke in stroke patients.
The results of this study were similar to those of previous studies. Balance was associated with strength and muscle mass, and ADLs were associated with grip strength [25-27]. These results suggest that SMI and grip strength are more important than physical performance in diagnosing sarcopenia. In Asian and Korean sarcopenia diagnostic guidelines, physical performance increases severity but is not required to diagnose sarcopenia [3,18].
The results of the present study are similar to those reported in previous studies. The stroke group with sarcopenia was found to be older than the group without sarcopenia [23]. Another previous study reported that the stroke group with sarcopenia had lower SMI and grip strength than the group without sarcopenia [28]. In the study by Park et al., the stroke group with sarcopenia also showed a tendency towards lower MMSE, FAC, and MBI compared to the group without sarcopenia [29]. These results suggest that the influence of stroke may act in con-junction with sarcopenia rather than independently. However, the pre-cise mechanism of sarcopenia in stroke patients remains uncertain. While stroke and sarcopenia are distinct chronic conditions, they mutually affect each other. When occurring together as comorbidities, patients experience a compounded burden, leading to notable declines in quality of life, increased rates of hospitalization and mortality, and great-er utilization of medical resources, among various outcomes [30].
Through this study, it was confirmed that SMI is associated with sarcopenia in stroke patients. Stroke patients exhibit a decrease in lean body mass compared to healthy adults [31]. The thigh muscle area and muscle mass of stroke survivors with hemiplegia are 20-24% lower than those of the non-paralyzed thigh [32]. Even when compared to healthy adults in terms of muscle size and strength, both paralyzed and non-paralyzed sides of stroke patients show deficiencies [33]. Therefore, it is believed that the reduction in SMI in stroke patients affects the diagnosis of sarcopenia. Stroke patients with insufficient muscle mass tend to have lower functional levels and longer hospital stays [34]. Stroke patients with sarcopenia have poor prognoses even with rehabilitation [29]. Therefore, interventions to increase SMI should be considered to prevent the diagnosis of sarcopenia in acute stroke patients.
The mechanisms of sarcopenia in stroke patients involve a complex interplay of various factors. Key mechanisms include immobilization and disuse atrophy, impaired feeding, sympathetic activation and inflammation, and denervation [30]. Therefore, continuous further research on sarcopenia in stroke patients is necessary.
This study is limited by the small sample size at one institution. Future studies should be conducted in more institutions and with a larger sample size. Additionally, there is a lack of data on the comorbidities of stroke patients and related patient characteristics, such as length of hospital stay. Future studies should provide detailed information about the characteristics of the study participants.
CONCLUSION
A high prevalence of sarcopenia was observed in acute stroke patients, and there were differences in characteristics between stroke patients without sarcopenia and those with sarcopenia. As sarcopenia factors increase, BBS and MBI also increase. Increasing SMI in acute stroke patients can reduce the risk of sarcopenia diagnosis; therefore, exercise interventions aimed at increasing SMI should be considered.
Notes
All authors declare that they have no conflicts of interest with the contents of this study.
AUTHOR CONTRIBUTIONS
Conceptualization: SY Kim, BG Kim; Data curation: SY Kim; Formal analysis: SY Kim, BG Kim; Funding acquisition: BG Kim, SJ Park; Methodology: SY Kim, BG Kim; Project administration: SJ Park; Visual-ization: BG Kim, SJ Park; Writing - original draft: SY Kim, BG Kim, SJ Park; Writing - review & editing: SY Kim, BG Kim, SJ Park