Exercise-Induced Laminar Blood Flow Maintains Vascular Function by Enhanced Endothelial Homeostasis
Article information
Abstract
PURPOSE
Laminar blood flow is known to play a critical role in maintaining endothelial homeostasis in blood vessels. It exerts a shear stress on the endothelial cells (ECs), which is essential for the regulation of various vascular functions. In this study, the effects of laminar blood flow-induced enhanced endothelial homeostasis on the maintenance of vascular function were investigated.
METHODS
Human umbilical vein endothelial cells (HUVECs) were employed to study gene expression associated with vessel dilation and angiogenesis in response to varying shear stress levels. To assess angiogenesis, experimental mice participated in voluntary wheel running for 16 weeks. To evaluate the vascular autophagic function, the six weeks old C57BL/6 mice underwent an hour of forced treadmill running for 10 weeks. The resulting gene expression was evaluated through western blotting and quantitative real-time polymerase chain reaction (qRT-PCR).
RESULTS
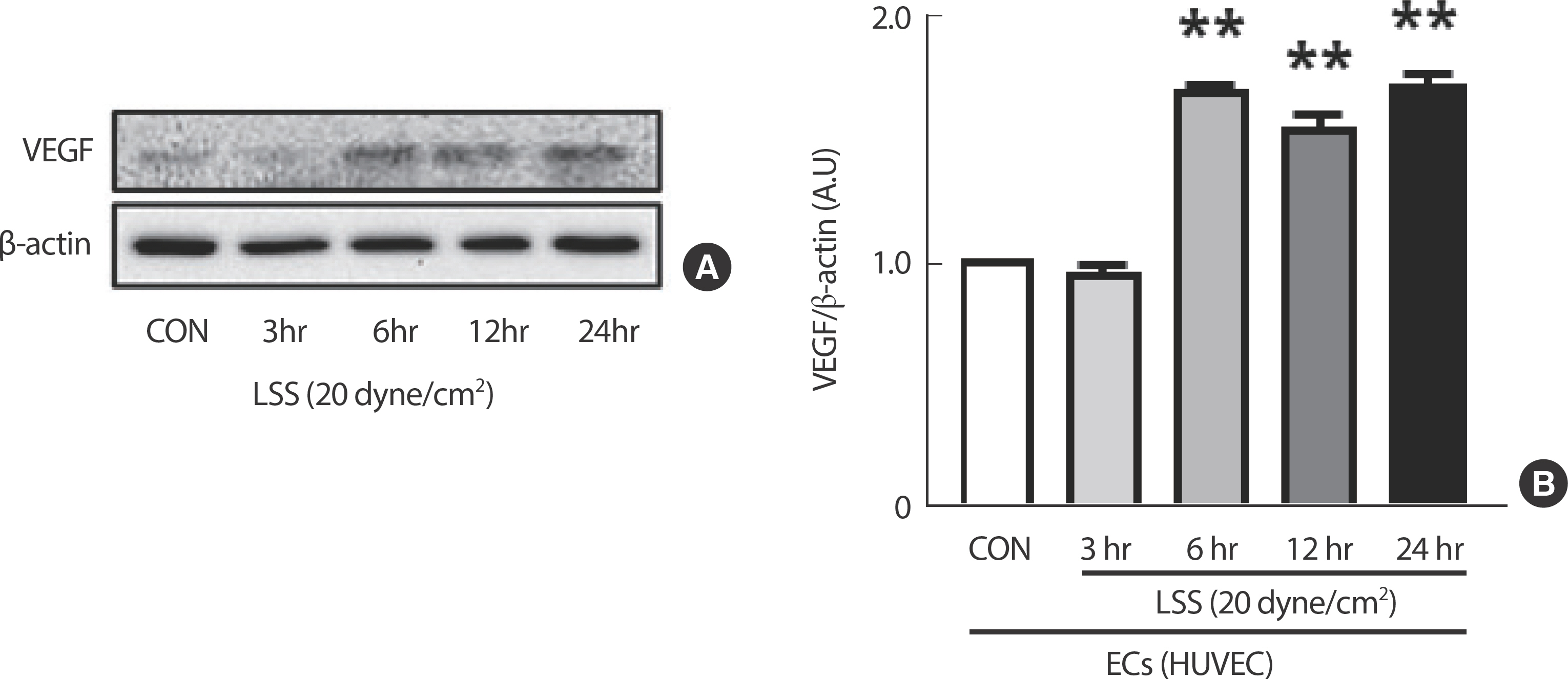
Disturbed flow resulting from partial ligation surgery in the mice's carotid artery led to a decrease in angiogenic capacity compared to the laminar flow observed in ex vivo sprouting assays. Cultured HUVECs exhibited a significant increase in VEGF expression when exposed to increased durations of 20 dyne/cm² laminar shear stress (LSS). Following a 10-week treadmill exercise regimen, ECs in the carotid arteries of mice showed a significant increase in the expression of autophagic genes, such as LC3II, Atg3, and Atg7, which was not observed in smooth muscle cells (SMCs). LSS was found to boost the expression of both total and phosphorylated eNOS, genes involved in vascular dilation regulation, specifically in ECs.
CONCLUSIONS
These findings suggest that laminar blood flow sustains vascular function, such as angiogenic capacity, through the enhancement of endothelial homeostasis.
INTRODUCTION
Cardiovascular diseases (CVDs) persist as a leading cause of global mortality, imposing significant burdens on healthcare systems and affecting individuals across diverse demographics [1,2]. Consequently, there is a growing interest in understanding the fundamental molecular mechanisms underlying the onset and progression of CVDs. Endothelial dysfunction, characterized by compromised vascular equilibrium and inflammation, emerges as a pivotal contributor to the pathogenesis of CVDs such as atherosclerosis, hypertension, and stroke [3].
The circulatory system operates as a complex network responsible for nutrient transport, waste elimination, and internal stability maintenance [4]. Central to this network are blood vessels, lined with endothelial cells (ECs), which play a crucial role in regulating vascular dynamics [5]. Among various factors shaping EC behavior, the nature of blood flow within vessels is of paramount importance. Laminar blood flow, characterized by a smooth flow of blood in parallel layers with consistent velocities, exerts uniform shear stress on ECs along vessel walls [6]. These cells act as key sentinels, forming a barrier between blood and vessel walls, and are vital for maintaining vascular function [7]. The shear stress induced by laminar flow is essential for preserving endothelial homeostasis and functionality [6,8].
Conversely, disrupted flow patterns, such as oscillatory or turbulent flows, trigger proinflammatory and prothrombotic responses within ECs [5]. These aberrant flow dynamics are closely associated with the development of dysfunctional endothelium prone to atherosclerotic plaque formation and vascular occlusion [9]. Understanding the conse-quences of disturbed flow on endothelial homeostasis provides valuable insights into the pathophysiology of CVDs, underscoring the importance of restoring laminar flow conditions to uphold vascular health.
ECs serve as a crucial interface between blood and surrounding tissues, orchestrating diverse functions including the regulation of vascular tone, leukocyte adhesion, and preservation of the blood-brain barrier [8,10]. Disruption of endothelial homeostasis represents a pivotal event in the pathogenesis of various CVDs, including atherosclerosis, hypertension, and stroke [3]. Therefore, unraveling the impact of laminar flow on endothelial homeostasis is of paramount significance in elucidating the mechanisms underlying vascular health and disease.
Laminar flow has been shown to modulate various signaling pathways within ECs, particularly enhancing nitric oxide (NO) production, a potent vasodilator crucial for maintaining vascular tone and blood pressure regulation [11-13]. Additionally, autophagy, a process involved in the degradation and recycling of damaged organelles and proteins, emerges as a critical mechanism in preserving endothelial homeostasis [14]. Dys-regulation of autophagy in ECs can lead to oxidative stress, inflammation, and endothelial dysfunction, contributing to the development of CVDs [15]. Furthermore, angiogenesis, a tightly regulated process facilitating the formation of new blood vessels from pre-existing ones, underscores the central role of ECs in various physiological processes [16].
The connection between exercise and ECs, as well as laminar blood flow, is a complex yet fascinating area of research in cardiovascular physiology. Exercise plays a crucial role in increasing shear stress and improving endothelial function [17]. This, in turn, enhances cardiac output and blood flow. The heightened shear stress stimulates the production of NO, a potent vasodilator that improves vascular function and lowers blood pressure. Furthermore, exercise-induced laminar shear stress (LSS) has an anti-inflammatory effect on ECs, reducing the expression of proinflammatory cytokines and adhesion molecules, which are crucial in the development of atherosclerosis [18]. Hence, exercise has significant effects on ECs mediated by LSS, leading to improved endothelial function, reduced inflammation, enhanced NO production, and vascular remodeling.
Despite acknowledging the mentioned fragmented understanding of each EC function within the vasculature, a comprehensive grasp of how exercise-induced enhancement of laminar flow impacts EC homeostasis and contributes to improving vascular health remains elusive. Understanding the intricate interplay between laminar blood flow and endothelial homeostasis is crucial for unraveling the mechanisms governing both vascular function and dysfunction. Therefore, this study aims to il-luminate novel therapeutic avenues for preserving vascular health and mitigating CVDs by investigating the molecular and cellular responses of ECs to aerobic exercise-induced shear stress.
METHODS
1. Animal care and exercise protocol
C57BL/6 mice were kept in a regulated environment with a temperature maintained in 23 o C, relative humidity between 50-60%, and subjected to a 12-hour light/12-hour dark cycle. They were provided with a standard diet and had continuous access to fresh water, both replenished daily. Euthanasia was performed under deep anesthesia induced by isoflurane to ensure the mice didn, t experience any discomfort during the procedure. Their welfare was prioritized by supplying fresh food and water at least twice a week, and changing bedding weekly to maintain a comfortable living space.
Mice in the voluntary wheel running group were housed individually in cages equipped with a wheel fitted with a digital magnetic counter. For the study determining angiogenic function, voluntary wheel running was performed from their 6th week to 22nd week of age for 16 weeks in total [19]. For the study to assess vascular autophagic function, treadmill running was performed in average 15 m/min, an hour/day, 5 days/week for 10 weeks including acclimation period [20]. According to the experimental concept and workflow, animal experiments were inte-grated with cell experiments (Fig. 1).
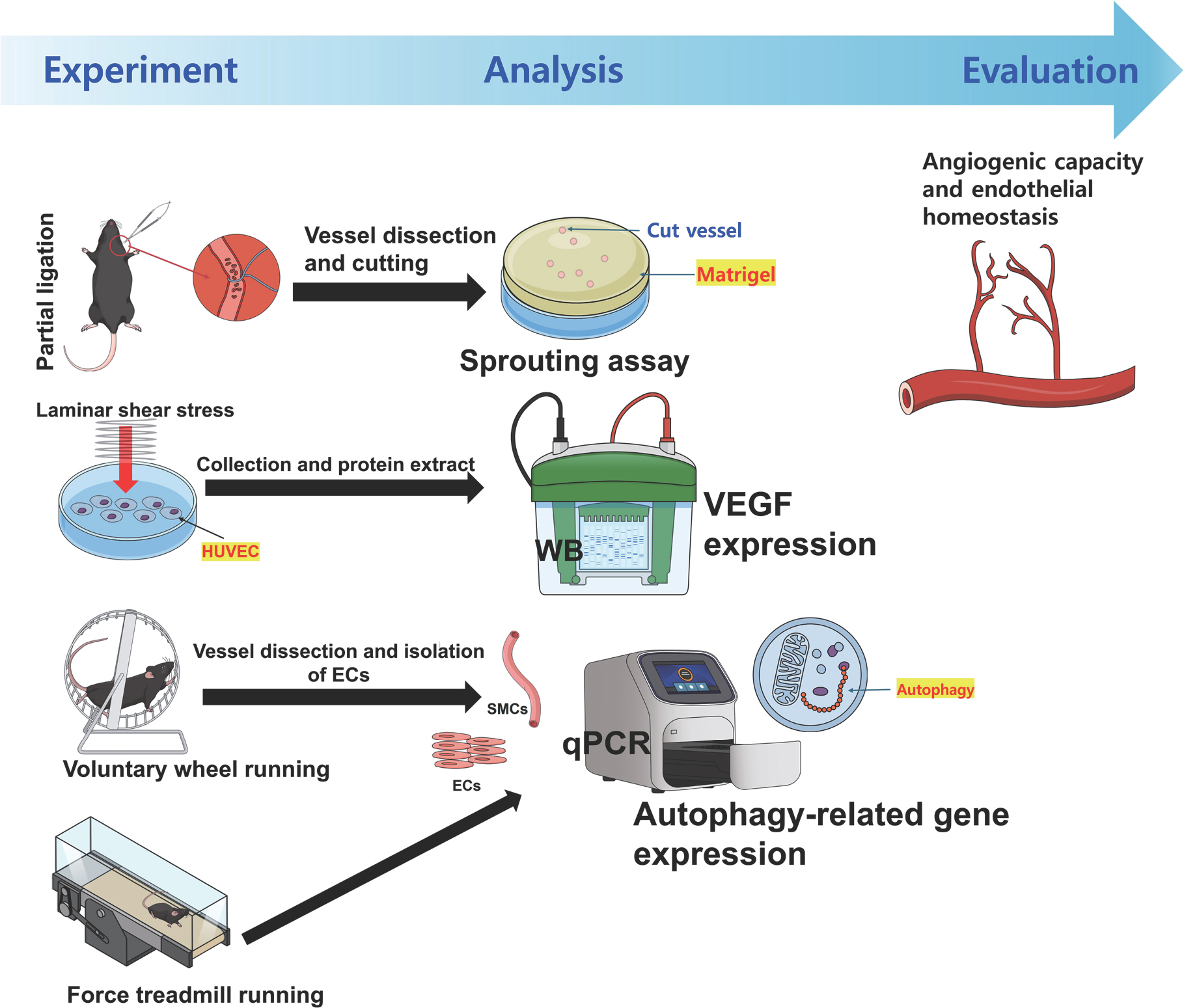
Study concept and workflow. WB, western blotting; HUVEC, human umbilical vein endothelial cells; ECs, endothelial cells; SMCs, smooth muscle cells; qPCR, real-time quantitative reverse transcription polymerase chain reaction.
All experimental procedures were conducted in accordance with ethical guidelines for laboratory animal care approved by the Institutional Animal Care and Use Committee (IACUC) with approval numbers PNU-2019-2448 and GNU-221117-M0158.
2. Carotid artery partial ligation
Carotid artery (CA) partial ligation surgery was performed to deter-mine the importance of laminar flow on the maintenance of vascular homeostasis compared to disturbed flow [21]. The partial ligation surgery on the left carotid arteries (LCA) of all twenty C57BL/6J male mice. Anesthesia was induced by an intraperitoneal injection of a xylazine (10 mg/kg) and ketamine (80 mg/kg) mixture. The epilated area was disinfected with betadine, and a ventral midline incision (4-5 mm) was made in the neck. the LCA was exposed by a blunt dissection. Three of four caudal branches of the LCA, such as the left external carotid (ECA), internal carotid (ICA), and occipital artery (OA), were surgically ligated with 6-0 silk suture leaving the superior thyroid artery (STA) as the only outflow. On the other hand, the right carotid artery (RCA) of the same mouse was left intact as the control. Then, the incision was closed with Tissue-Mend (Veterinary Product Laboratories). Mice were monitored until recovery in a chamber on a heating pad following surgery.
3. Carotid artery extraction and angiogenic sprouting assay
To assess angiogenic potential, an ex vivo aortic ring assay was conducted utilizing the common carotid artery (CCA). Following anesthesia with isoflurane, the left and right CCAs were extracted four weeks post-partial ligation surgery. An incision was made from the sternal region to the neck, followed by a midline incision (4-5 mm) to expose the carotid arteries. The entire left and right CCAs were excised and promptly placed in a sample tube containing pre-warmed cell growth medium (EGMTM-2 Endothelial Cell Growth Medium-2 BulletKitTM, #CC-3162, Lonza, Basel, Switzerland) in a water bath at 37o C. Subsequently, each CCA in the sample tube was divided into six equal rings of 15 μm thickness in a petri dish. Matrigel Matrix (Corning, Bedford, MA, USA) was dispensed onto the bottom of a 24-well plate and incubated at 37 o C to polymerize 15 minutes prior to dividing the CA ring. The prepared CA ring was then implanted into the Matrigel Matrix to form an annular shape. Cell growth media were added to each well, and the plate was cultured in an incubator (37 o C, 5% CO2). After one week of culture, the number of branches protruding from the CCA ring was quantified using a cell imaging system (EVOS M5000, Thermo Fisher Scientific, Carlsbad, CA, USA) [19].
4. Endothelial and smooth muscle cell isolation from carotid artery
Following anesthesia with isoflurane, an incision was made from the sternal region to the neck. The entire length of both the left and right subclavian common carotid arteries (CCAs) was harvested. Subsequently, the CCAs were promptly flushed for a few seconds using a 29-gauge insulin syringe filled with QIAzol Lysis Reagent (QIAZEN Sciences, Maryland, USA) and transferred into a microfuge tube. The eluate in the microfuge tube contained ECs. The remaining CA tissue, post-lumen flushing, represented SMCs [19]. These collected samples were ear-marked for quantitative real-time polymerase chain reaction (qRT-PCR) analysis.
5. Cell culture
Human umbilical vein endothelial cell (HUVEC) lines from Lonza were grown in M199 medium supplemented with 20% fetal bovine se-rum and endothelial cell growth supplement (Sigma-Aldrich no. E2759), and maintained at 37°C in a 5% CO2 atmosphere. All experiments with HUVEC were conducted between passages 3 and 5. For shear experiments, HUVECs, grown to 90-100% confluence, were replaced with shear media and exposed to the physiological levels of shear stress for various times using orbital shaker in 20 dyne/cm².
6. Quantitative real-time RT-PCR (qPCR)
Following the homogenization of ECs and SMCs utilizing an ultrasonicator (VCX-400, Vibra Cell, Sonics & Materials Inc., Danbury, CT, USA), total RNA extraction was performed using TRIzol reagent (Ambion, Carlsbad, CA, USA). Subsequently, complementary DNA (cDNA) was synthesized from the RNA using AccuPower1 RT Premix (Bioneer, Dae-jeon, Korea) in accordance with the manufacturer's protocol. The synthesized cDNA was then combined with forward and reverse primers for quantitative polymerase chain reaction (qPCR) in a 384-well plate (Micro-Amp1384-Well Reaction Plate with Barcode, Applied Biosystems, Foster City, CA, USA) containing Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). PECAM and α-SMA were chosen as markers to distinguish between EC (higher PECAM expression than α-SMA expression) and SMC (higher α-SMA expression than PECAM expression). To standardize mRNA levels across samples, GAPDH was ampli-fied by qPCR as a reference gene. The primer sequences utilized in the experiments are provided in Table 1. Subsequently, the output cycle thresh-old (Ct) values were quantified.
7. Western blotting
ECs were subjected to homogenization in ice-cold RIPA lysis buffer supplemented with protease inhibitor (Roche no. 11836153001). The proteins extracted from cell lysates were separated using Tris-glycine SDS PAGE and subsequently transferred to an Immobilon-P PVDF mem-brane for standard ECL Western blotting. Chemiluminescence was employed to detect total protein expression. Band densitometry analyses were conducted utilizing Image J software (National Institute of Health) to facilitate comparisons between experimental groups. The values generated by Image J were represented in arbitrary units. The densities of the specific protein bands were normalized to the densities of the internal control genes.
8. Statistical analysis
Data are presented as mean±SE. Differences across experimental conditions were assessed by t-test and analysis of variance followed by post-hoc testing with the Fisher's least significant difference test. p <.05 was considered statistically significant for all analyses.
RESULTS
1. Disturbed flow dysregulates aortic angiogenic function, while laminar flow maintains
Partial ligation causing disturbed flow on LCA led to a significant decrease in the sprout number compared to the RCA which kept experiencing laminar flow (Fig. 2).
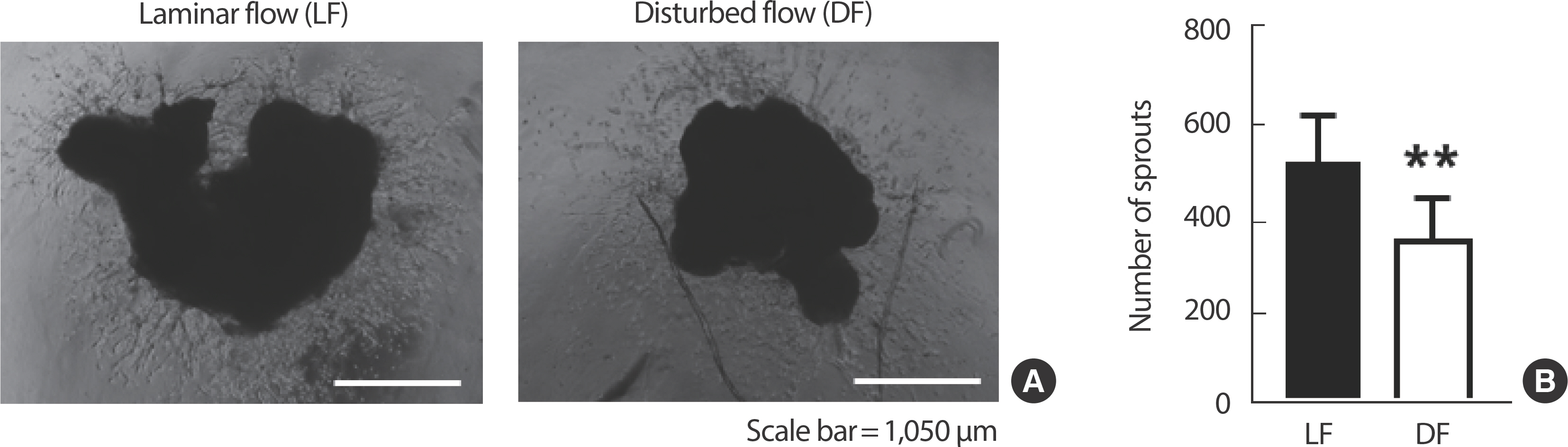
Laminar flow maintains aortic angiogenic function, while disturbed flow dysregulates. (A) 10× magnification microscopic images of angiogenic sprouting assay. (B) Comparison of the number of sprouts extending after culture of isolated common carotid arteries. All data are presented as the mean ±SE. LF, laminar flow; DF, disturbed flow. ** p<.01 compared to LF.
2. Increased LSS boosts the expression of angiogenesis regulator genes in ECs but not in SMCs
The expression of the angiogenesis regulator gene VEGF in HUVEC was significantly increased by a clinically verified shear intensity during exercise, 20 dyne/cm², in a time-dependent manner (Fig. 3).
3. AEX-induced enhanced LSS elevates the expression of autophagy-related genes in ECs but not in SMCs
After a 10-week treadmill exercise regimen, there was a significant increase in the expressions of autophagy-related genes such as LC3II, Atg3, and Atg7, accompanied by a significant decrease in p62 expression, indicating the activation of autophagy in isolated C57BL/6 mice ECs (Fig. 4B). However, there were no observable changes in autophagy in SMCs (Fig. 4C). The distinction between EC and SMC was confirmed using PECAM and α-SMA markers, with higher PECAM expression in ECs than α-SMA and vice versa in SMCs (Fig. 4A).
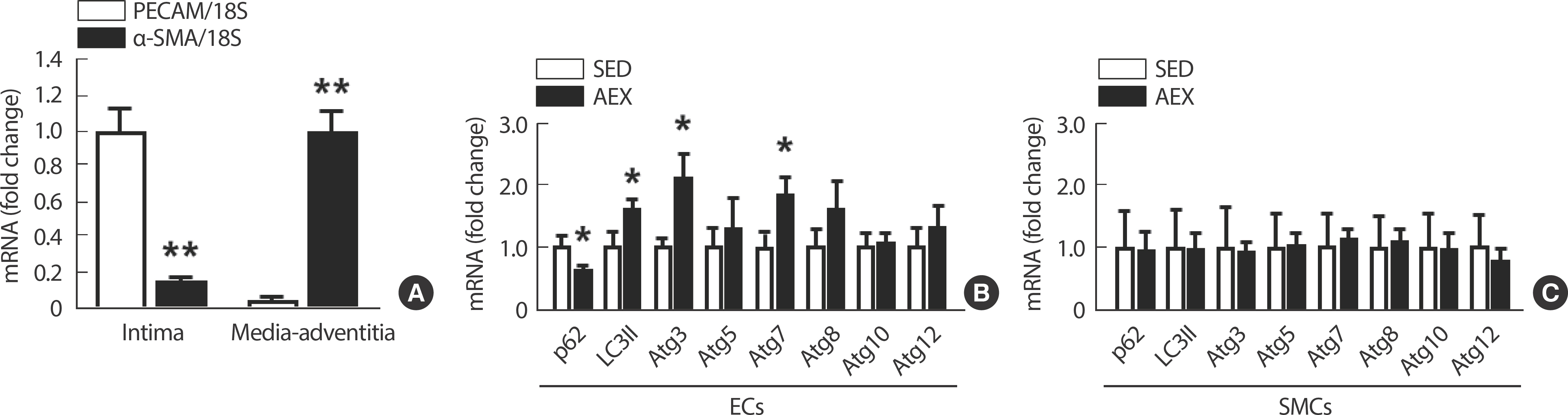
AEX-induced enhanced LSS elevates the expression of autophagy-related genes in ECs but not in SMCs. (A) Difference in the expressions of PECAM and α-SMA in isolated vascular ECs and SMCs. (B) Autophagy-related gene expressions according to SED and AEX in ECs. (C) Autophagy-related gene expressions according to SED and AEX in SMCs. All data are presented as the mean±SE. SED, sedentary; AEX, aerobic exercise. * p<.05 compared to SED, ** p<.01 compared to PECAM.
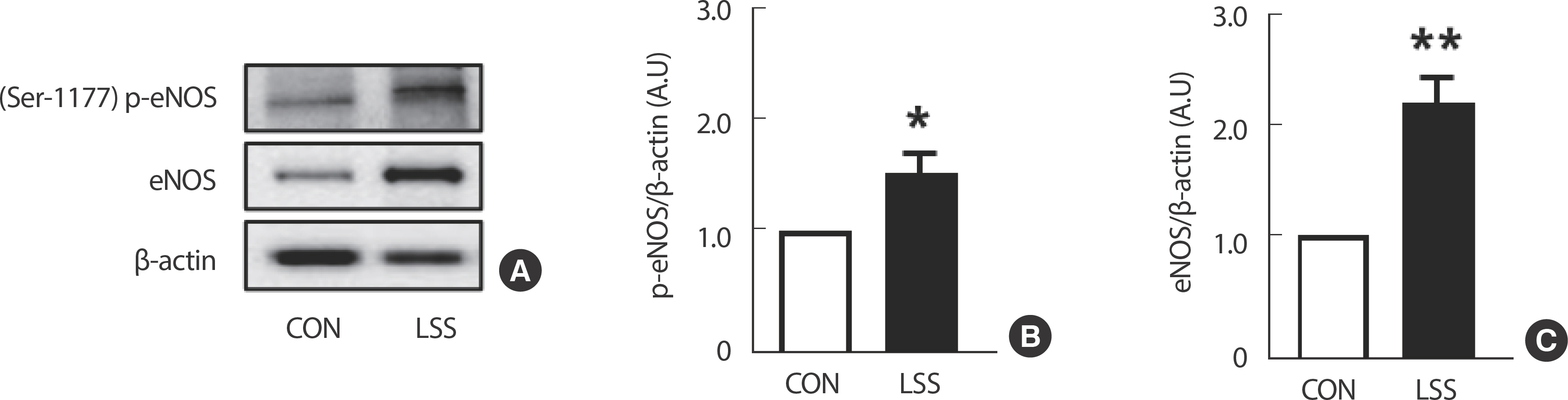
4. LSS enhances the expression of both total and phosphorylated eNOS, which are genes regulating vascular dilation, in ECs
Exposing HUVECs to 20 dyne/cm² of LSS for 24 hours resulted in a significant upregulation of Ser-1177 p-eNOS and total eNOS expressions, both recognized as biomarkers of vasodilation (Fig. 5).
DISCUSSION
CVDs pose a major global health challenge, highlighting the importance of gaining a thorough comprehension of their fundamental molecular mechanisms to formulate efficient preventive and therapeutic approaches [1]. Endothelial dysfunction, marked by disruptions in vascular balance and inflammatory processes, emerges as a prominent factor contributing to a range of CVDs, such as atherosclerosis, hypertension, and stroke [22]. The maintenance of endothelial homeostasis is critical for vascular health, and emerging evidence suggests that the nature of blood flow within vessels plays a pivotal role in regulating endothelial function [23].
Laminar blood flow, characterized by smooth and consistent blood flow patterns exerting uniform shear stress on ECs, emerges as a crucial factor in maintaining endothelial homeostasis [24]. In contrast, disturbed flow patterns, such as oscillatory or turbulent flows, trigger proinflammatory and prothrombotic responses within ECs, contributing to endothelial dysfunction and the development of CVDs [25]. Our study aimed to elucidate the molecular mechanisms underlying the influence of laminar flow on endothelial homeostasis and vascular function.
We employed both in vivo and ex vivo experimental models to investigate the impact of laminar flow on endothelial homeostasis and vascular function. Utilizing a partial ligation model in mice, we demonstrated that disturbed flow induced by partial ligation of the CA resulted in impaired angiogenic function compared to vessels experiencing laminar flow (Fig. 2). This finding underscores the importance of laminar flow in preserving endothelial function and vascular health [26].
Furthermore, our study revealed that laminar flow enhances the expression of angiogenesis regulator genes in ECs, highlighting its role in promoting vascular remodeling and angiogenesis (Fig. 3) [27]. Additionally, we observed that laminar flow-induced aerobic exercise significantly upregulated autophagy-related genes in ECs, indicating the activation of autophagy, a crucial mechanism for maintaining endothelial homeostasis (Fig. 4) [28]. Notably, these effects were not observed in SMCs, emphasizing the specificity of laminar flow-mediated responses in ECs [29]. Based on findings from prior research indicating that enhanced autophagy function correlates with improvements in processes like angiogenesis [30], it is hypothesized that the enhancement of autophagy function in ECs also played a positive role in the improvement of vascular function observed in this study.
Moreover, our findings indicate that laminar flow enhances the expressions of phosphorylated and total eNOS, a key regulator of vascular dilation, in ECs (Fig. 5) [31]. This suggests that laminar flow contributes to the maintenance of vascular tone and blood pressure regulation by promoting vasodilation through increased NO production.
Our research indicates that when the body, s vascular homeostasis is disrupted and blood flow is impaired, the growth of angiogenesis is hin-dered. However, we also found that the shear stress experienced by vascular ECs during physical activity can promote angiogenesis. These findings suggest that exercise is important for maintaining a homeostasis in blood vessels. Additionally, the positive effects of exercise on the activation of genes related to autophagy and the release of NO from vascular ECs, as observed in experimental animals, highlight the importance of LSS within human blood vessels that changes during exercise. We recognize that our study may have primarily focused on the vascular benefits of aerobic exercise, potentially overlooking its broader physiological advantages for the human body. Furthermore, while exercise provides various benefits for blood vessels, our emphasis may appear to be solely on factors related to vascular ECs and autophagy. These limitations should be acknowledged. However, considering the significant impact of circulatory system imbalances on the human body, changes in blood vessels are crucial. Vascular ECs and autophagy play essential roles in maintaining blood vessel homeostasis. Therefore, despite the study, s limitations, we believe that the results provide valuable insights for future research in exercise physiology.
Despite being limited to verifying vascular function solely through angiogenesis, this study provides valuable insights. It suggests that enhancing LSS through aerobic exercise contributes to maintaining and strengthening vascular function by improving various EC functions and enhancing EC homeostasis. Despite this constraint, the study, s integrat-ed approach and findings regarding the beneficial effects of exercise on vascular health make it significant.
CONCLUSIONS
In conclusion, this study provides valuable insight into the molecular mechanisms underlying the protective effects of exercise-induced or in-dependent laminar blood flow on endothelial homeostasis and vascular function and provides additional knowledge about the diverse effects of exercise on blood vessels. Further research is warranted to explore the therapeutic potential of interventions targeting laminar flow-mediated pathways in the prevention and management of CVDs.
Notes
The authors declare that they do not have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: JS Kim, Y Park; Data curation: JS Kim, KW Baek, SJ Kim; Formal analysis: JS Kim, KW Baek, SJ Kim, J Lee; Funding ac-quisition: JS Kim; Methodology: JS Kim, KW Baek, Y Park; Project administration: JS Kim, Y Park; Visualization: JS Kim; Writing-original draft: JS Kim; Writing – review & editing: Y Park, J Lee, CA Johnston.