INTRODUCTION
METHODS
RESULTS
1. ECM as a biological scaffold
2. Satellite cells as sources of muscle regeneration
3. Myofibers as a source of satellite cell
4. Resistance training and muscle regeneration
Fig. 1.
Fig. 1.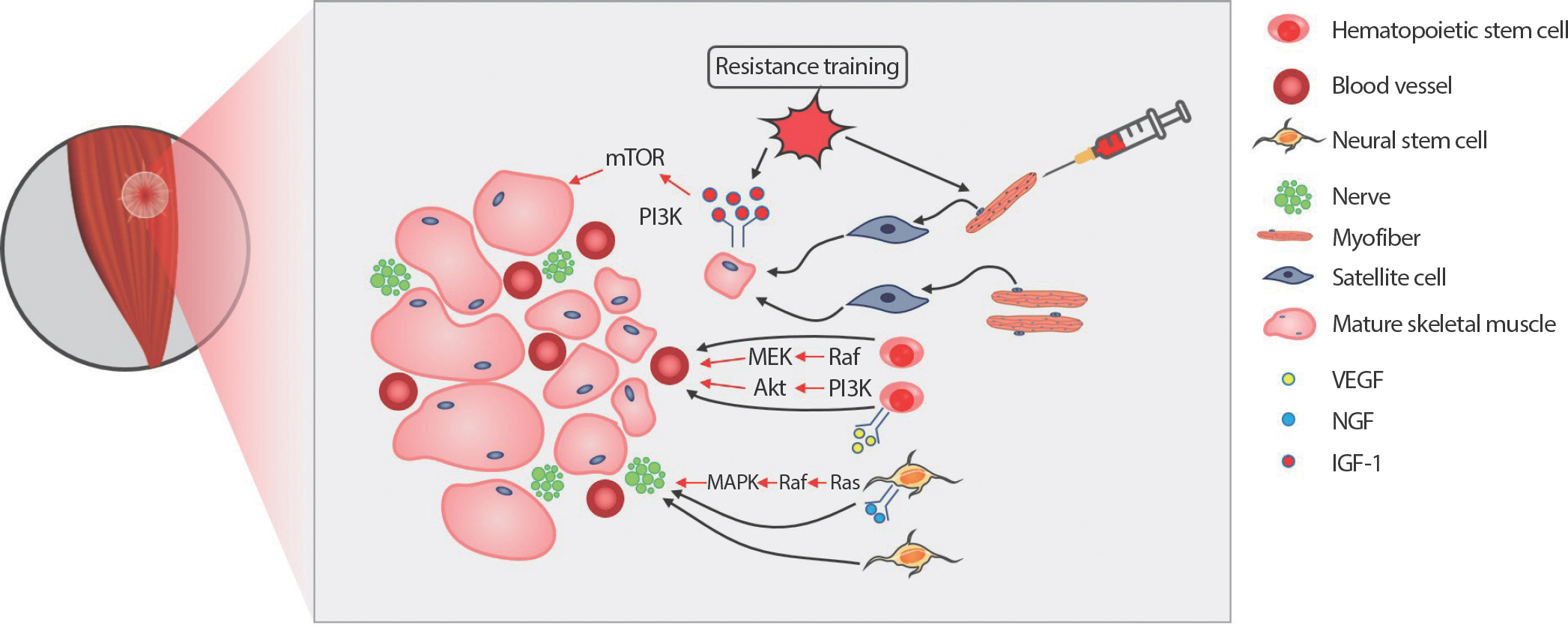
AbstractPURPOSEMinor skeletal muscle injuries can be repaired, but more extensive volumetric muscle loss (VML) leads to a permanent functional disability with ambiguous therapeutic outcomes, and reconstructive surgical procedures are constrained by donor tissue scarcity. This review assessed the considerable attention paid to biomaterials in healing damaged skeletal muscle.
METHODSA comprehensive search in PubMed, Web of Science, Google Scholar, and Wiley Online Library was conducted to obtain previous studies exploring the state of biocompatible tissue scaffolds for VML recovery.
RESULTSBy regenerating the function of damaged skeletal muscle, tissue-engineered skeletal muscle construction could revolutionize the treatment of VML. However, transporting cells into the wounded muscle location presents a significant challenge because it may result in unfavorable immunological reactions. The development and validation of several biomaterials with varying physical and chemical natures to treat various muscle injuries have recently been undertaken to overcome this problem. This review discusses the relative benefits of satellite cells (SC), the most prevalent skeletal muscle stem cells employed to seed scaffolds.
CONCLUSIONSBiomaterials can be used with skeletal muscle stem cells and growth factors to repair VML because of their customizable and desirable physicochemical qualities. Owing to the capacity of SCs for self-renewal and their undifferentiated state, these cells are excellent candidates for cell therapy. A large gap exists between understanding SC behavior and how it can be used to repair and regenerate human skeletal muscle tissue. Thus, this review sought to portray the current knowledge on the lifespan of SCs and their involvement in exercise-induced muscle regeneration and hypertrophy.
INTRODUCTIONLarge-volume skeletal muscle loss usually results in functional deficits, cosmetic flaws, emotional pain, and a permanent disability [1]. Volumetric muscle loss (VML) occurs due to motor vehicle accidents and surgical ablation, including tumor excision and diabetic foot debridement in civilians and gunshots or explosive-related injuries in military personnel. Severe full-thickness muscle loss creates a large gap between the end of the transected muscle stumps that hampers the natural regenerative process and consequently triggers fibrosis [2], which obstructs myofiber ingrowth and blood vessel infiltration because of the existing connective tissue structure. As a VML injury results in muscle loss and damages to nerves, blood vessels, and the extracellular matrix (ECM), the completion of functional and morphological repair remains a big challenge. Therefore, the treatment of VML injuries should simultaneously target enhanced myogenesis and inhibition of fibrosis.
Appropriate scaffolding provides a space for the ingrowth of new myofibers which can inosculate with the remaining myofibers. Biological scaffold transplantation using myogenic cells is a research hotspot in regenerative medicine. Surgical free-muscle transplantation is a standard treatment for VML and often causes not only donor-site morbidity and functional deficits, but also is unsuitable for areas with large-volume muscle tissue removal [3,4]. Thus, the transplantation of synthetic scaffolds constitutes an attractive approach because of the high reproducibility and availability despite adverse effects, including excessive inflammatory response and failure to incorporate into host tissues [5].
Alternatively, acellular ECM can facilitate tissue repair in tissues, such as cardiac muscles, skeletal muscles, and the abdominal wall [6,7]. Acellular ECM preparation involves several processes, such as tissue harvesting, decellularization, and sterilization. Nonetheless, the residual small amount of DNA in acellular ECM confers a risk of immunological rejection of xenogenic scaffolds. However, ECM component proteins are seemingly highly conserved across species [8]. In a clinical study, the transplantation of acellular porcine ECM to patients with skeletal muscle injury of the quadriceps resulted in the formation of new muscle tissues after 36 weeks post-transplantation and even evoked remarkable contractile properties [9].
Compared with no treatment, there is emerging evidence that diverse treatments/remedies for VML can ameliorate muscle recovery and function. However, the favorable impact of the therapies on muscle regeneration from VML is uncertain. Thus, novel therapeutic treatment paradigms must be developed to provide clinically appreciable changes in muscle functional capacity. This brief review summarizes the clinical outcome of acellular biomaterials or biological ECM in the absence or presence of satellites cells, progenitor cells, and growth factors that enhance functional capability after VML damage.
METHODSUsing online databases such as Pubmed, Web of Science, Google Scholar, and Wiley Online Library, previous studies on the VML and potential therapeutic interventions were collected and analyzed for this review study. The web databases were searched using terms including “ satellite cells”, “ skeletal muscle regeneration”, “ VML injury”, “ ECM scaffold”, “ resistance training”, “ angiogenesis”, and “ innervation” for data collection.
RESULTS1. ECM as a biological scaffoldThe ECM is a three-dimensional structure that consists of glycoproteins, heparin sulfate proteoglycans, glycosaminoglycans, and type IV collagen, all of which play a crucial role in skeletal muscles function and structural support [10]. The ECM communicates with cells through integrin subunits to regulate the activation, proliferation, and differentiation of progenitor cells [11]. The activity of myogenic cells depends heavily on ECM components, especially primary ECM components such as collagen, laminin, and fibronectin, have been investigated extensively. Agarose hydrogel scaffolds coupled with laminin promotes neurite extension from the dorsal root ganglia [12]. Bioengineered hydrogel, when used with collagen and fibrin, increase functional properties and induce cellular hypertrophy [13]. In contrast, the inhibition of collagen synthesis hampers myoblast differentiation [14]. Myogenic cells that are cultured on dishes coated with ECM component proteins promote myoblast proliferation, and differentiation, especially with regard to satellite cells (SC) that adhere to laminin and increase motility [15–17]. Duchenne’ s muscular dystrophy (DMD), which is characterized by an inability to produce dystrophin that makes the sarcolemma brittle, illustrates the importance of ECM proteins in muscle structure and function. Patients with DMD experience progressive muscle degeneration that culminates in premature death.
ECM elasticity is one of the factors that determine stem cell lineage specification. Mesenchymal stem cells (MSCs) cultured on collagen-coated plates with different elasticity, to mimic the stiffness of the original tissues, differentiate into neural tissues on a soft plate, into myogenic tissues on a medium-stiff plate, and into bones on a rigid plate [18]. MSCs cultured on a stiff surface express smooth muscle cell markers, whereas MSCs cultured on a soft surface express chondrogenic markers. Moreover, stem cell proliferation depends on tissue stiffness. The ratio of MSC proliferation in response to transforming growth factor-β (TGF-β) depends on the matrix stiffness [19].
Furthermore, the ECM serves as a reserve of growth factors and binds to and releases growth factors [20], including fibroblast growth factor (FGF), TGF-β, hepatocyte growth factor (HGF), and VEGF. During the repair phase of muscle regeneration, chemokines that are released from ECM degradation attract progenitor cells and stem cells [21,22]. After injures, macrophages and SCs release matrix metalloproteinases (MMPs) that promote the secretion of growth factors which bind to the ECM. The ECM-secreted growth factors enhance progenitor cell migration and proliferation [20,23,24]. Cultured SCs under high TGF-β1 signaling inhibit the MyoD and myogenin expression resulting in limited SC proliferation and differentiation [25]. Single myofiber studies show that HGF and FGF-2 affect SC proliferation [26].
Acellular ECM has significant benefits as a scaffold because antigens, which can trigger an immune response, are removed, and tissue remodeling macrophages are promoted by acellular ECM transplantation [27]. Another benefit of acellular ECM is biocompatibility and biodegradability, which induces host cells to self-produce matrix [28]. Furthermore, the 3D structure of ECM retains the original structure of myofibers, vessels, and nerves, which facilitates myotube alignment and incorporates nerves and blood vessels into the ECM [28]. Acellular ECM scaffolds seeded with progenitor cells have been extensively researched in VML injuries [28,29]. Blood vessels and myofibers have been displayed in an implanted homologous muscle acellular matrix [30]. The implantation of acellular ECM along with myoblasts can repair abdominal wall defects [31], whereas only homologous acellular ECM implantation resulted in a large volume of fibrous scar tissues [32]. Previous studies have shown the ability of the homologous acellular ECM to support muscle and blood vessel regeneration [33] where implanting an acellular ECM seeded with MSCs improves functional recovery as well [29].
2. Satellite cells as sources of muscle regenerationSCs, which are adult stem cells, are primary resources for skeletal muscle regeneration. SCs that are located between the basal lamina and sarcolemma are mitotically quiescent. A hallmark of SC is the self-renewing capacity that has been demonstrated in SCs transplantation studies. Transplantation of SCs into SC-depleted MDX mice with a mutation in the mouse dystrophin gene induced myofiber formation, migration to the host SC niche, and transplanted SCs were are incorporation with host fibers [34]. The number of SCs in a single fiber is associated with age and fiber types, and SCs are unevenly distributed along with fiber. 30-35% of nuclei in a postnatal mouse express SC markers while an adult mouse consists only 1-4% of SC [35]. Soleus, a slow type of muscle, has two- to three-fold higher number of SCs than extensor digitorum longus, a fast type of muscle [36,37]. The population of SC is heterogenous in mammalians such as mice, rats, and humans [38–40]. A high density of SC has been observed adjacent to capillaries [41] and close to the motor neuron junction [42], which implies that they contribute to SC character. SCs are characterized by distinct markers in each sequential step. Quiescent SCs are characterized by Pax7, paired box7, which are crucial in SC development and lineage determination. Pax7- null mice appear 50% decrease in body weight at 7 days of age, and even pax7-null mice die within 2 weeks after birth [43,44].
Growth factors play a major role in SC proliferation and differentiation. Insulin-like growth factor-1 (IGF-1) is a well-known growth factor for skeletal muscle regeneration and can change the expression of myogenic regulatory factors and upregulate SC proliferation [45,46]. HGF leads SC migration to the site of damage [47] and SC proliferation by binding to c-met [48]. SCs exposed to muscle injury sites start proliferating by multiple cellular and molecular responses governing the SC environments during muscle regeneration processes. Activated SCs, often referred to as myoblasts, are characterized by the upregulation of Myf5 and myoblast determination protein 1 (MyoD) [49–51]. MyoD upregulation relates to SC proliferation, and SC differentiation depends on MyoD downregulation. MyoD-null mice have shown a limited regenerative capacity characterized by abnormal myoblasts morphology [52] and decreased the number of regenerating myofibers [53]. When SCs enter the differentiation phase, both myogenin and Myf 6 are highly expressed [54,55]. Myogenin is essential for the fusion of myoblasts, either among themselves or with existing myofibers. These small myofibers become mature fibers as they grow in size and express contractile proteins, such as actin and myosin. In contrast, myogenin-null mice have only a few myofibers at birth, and they die prenatally [56–58].
The probability of SC transplantation for muscle regenerative medicine has been extensively studied. Donor cells-derived myofibers are investigated by donor gene expression in host tissues [59,60]. Donor SCs not only fuse with themselves or host myofibers, but also exist as SCs within regenerating myofibers through self-renewal [34,61].
3. Myofibers as a source of satellite cellSCs are the primary sources for skeletal muscle regeneration. However, enzymatically dissociated SC transplantation has the challenge of resolving skeletal muscle wasting disorders [62,63]. Enzymatically dissociated and transplanted SCs induce incomplete muscle regeneration due to limited migration of SC, donor–host histocompatibility, and early death of donor SCs. Individual, freshly isolated single myofiber transplantation is an alternative strategy to deliver SCs to damaged muscles and maintains SC stemness because SCs are influenced by the original environment, thereby increasing the survival ratio of donor SCs. Individual myofiber contains 7-22 SCs in a mouse [44,51], and SCs migrate from the fiber to the damaged site. Single myofiber containing 7 SCs generated more than 100 new fibers containing 25,000-30,000 myonuclei [59]. A single donor myofiber promotes muscle hypertrophy and an increase in cross sectional area (CSA) [64]. Single myofiber transplantation results in hypertrophy with high force production, and donor fiber-derived SCs in young age remain in a host muscle permanently, which prevents age-related mass reduction [65]. In a previous study in our labo-ratory, transplanting 50 myofibers into preembedded ECM increased the number of regenerating fibers and decreased decellularized areas in a VML rat.
4. Resistance training and muscle regenerationResistance training (RT) influences functional properties of skeletal muscles by modifying fiber structure, mass, metabolism and promoting the release of growth factors. The intensity and mode of exercise induce specific adaptation of skeletal muscles. RT potentially increases muscle mass and contractile properties. After 6 weeks of heavy RT, muscle strength increases by 15% [66]. RT enhances the muscle protein synthesis ratio in humans by 50% at 4 hours after heavy resistance training [67]. RT contributes to myofiber hypertrophy and skeletal muscle regenerative capacity [68,69]. SC increases by 17% after extreme 16-week RT [70]. Powerlifters with a higher SC volume respond to injuries acutely [71].
RT is one of the best treatments for VML injury because VML results in severe functional deficits. 10% of the mass of the muscle loss results in 30% of initial peak isometric force reduction [72]. When 20% of the mass of the tibialis anterior (TA) muscle is excised, the TA exhibits 29% and 31% of a functional deficit in 2 and 4 months post-injury [73]. Therefore, VML patients require special treatment to enhance functional recovery. Low-intensity exercise is beneficial to repair the cardiac tissues [74], and high-intensity exercise attracts circulating mesenchymal stem cells into the damaged tissues in myocardial ischemia patients [75]. Both resistance and endurance training trigger angiogenesis and vascularization [76,77]. It is particularly important to have vascularization when repairing or regenerating tissues to attract circulating and residence cells. Blood vessels provide nutrients and oxygen to support muscle reconstruction [78]. Collectively, as aforementioned, therapeutic effects of donor SC and resistance exercise on skeletal muscle regeneration from VML are depicted in Fig. 1.
Fig. 1.Fig. 1.Effect of resistance exercise and donor satellite cell (SC) on skeletal muscle regeneration from volumetric muscle loss (VML). Transplanted extracellular matrix (ECM) fills the gap and roles as a scaffold to infiltrate circulating cells for skeletal muscle regeneration. Resistance exercise activates SC from the single donor myofibers. Resistance exercise also stimulates insulin-like growth factor-1 (IGF-1) downstream signaling pathways, accelerating protein synthesis, and preventing protein degradation. The upregulation of the IGF-1 downstream pathways contributes to the maturation of recently regenerated small muscle fibers. Vascular endothelial growth factor (VEGF) signaling pathway initiates hematopoietic stem cells proliferation and differentiation process into angiogenic cells, which is critical for vasculogenesis and angiogenesis. Nerve growth factor (NGF) binds to tropomyosin receptor kinase A (TrkA) and actives Ras-mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)-Akt signaling pathways to promote the survival and differentiation of neural stem cells. Successful innervation and vascularization are crucial steps for functional and structural completion of skeletal muscle regeneration in VML. 
5. Innervation and vascularization of the ECMNew myofiber formation should synchronize with vascularization and innervation together because of the role of blood vessels and nerves in skeletal muscles. Transmission of nerve impulses causes skeletal muscle contraction. A nerve fiber consists of axons, and myelin sheath develops central and peripheral nerves. Motor and sensory nerve fibers involving peripheral nerves directly regulate muscle contraction. Innervation plays a vital role in functional morphological skeletal muscle maintenance [79]. Denervated skeletal muscles continue the path of degeneration due to the failure of neuromuscular junctions, resulting in atrophy and tissue necrosis [80]. Skeletal muscle regeneration occurs following denervation, but this process cannot continue when SCs are depleted [79]. As denervation results in adverse effects on the muscle, regenerating myofibers should accompany innervation in a VML injury. Schwann cells, the primary glia of the peripheral nerve fiber, induce nerve regeneration and muscle innervation.
Activated Schwann cells secrete cytokines and neurotrophic factors that guide axon growth [81]. Innervation by axons depends on the remaining Schwann cell tubes and Schwann cell extension processes leading to end plates [82]. The presence of motor neurons affects Schwann cell viability. Motor neurons secret neuregulins, which are glial growth factors that promote survival and proliferation of Schwann cells [83,84]. The effects of neuregulin on muscle through binding tyrosine kinase receptors have been observed. Human myoblasts cultured with neuregulin express an increase in acetylcholine receptors and myosin heavy chains [85]. Other studies have researched the role of neurotrophic factors such as nerve growth factor (NGF), neurotrophin-3 (NT-3), and brain-derived neurotrophic factor (BDNF) on skeletal muscle regeneration. NGF is necessary for the survival and growth of neurons. Phenotypic characterization of NGF knockout mice increases in cell death and muscle dystrophy [86]. NGF treated with laminin and muscle-derived stem cells enhance neurite extension and engraftment efficiency, respectively [12,87]. NT-3, which is a neurotrophic factor that encourages nerve growth, modulates neurogenesis. The NT-3-overexpressing transgenic mice increase sensory and motor neurons, and NT-3 delivery enhances axonal regeneration in denervated muscle [88]. BDNF supports the survival of existing neurons and promotes the growth and differentiation of new neurons [89,90]. BDNF also influences skeletal muscle regeneration. BDNF depleted mice display a decrease in Pax7 positive cells, as well as impair skeletal muscle regeneration. These studies highlight the interaction between nerve growth and skeletal muscle cells in skeletal muscle regeneration.
Blood vessels supply nutrients and oxygen to muscle tissues. The diffusion distance of oxygen and nutrients from microvessels to cells is only limited to 150-200 µm [91]. Even though immediate hypoxia condition triggers endothelial cell proliferation [92], muscle tissues cannot survive without blood supply in the long term. Endothelial cell (EC)s located on the interior surface of blood vessels are involved in vascular biology. EC transplantation in myocardial injury stimulates angiogenesis, which increases regional perfusion [93]. Vascular endothelial growth factor (VEGF) is a primary regulator of angiogenesis. VEGF-derived angiogenesis promotes skeletal muscle regeneration. VEGF overexpression via virus gene transfer shows an increase in forelimb strength and a decrease in necrotic fibers area in mdx mice [94]. The combined delivery of VEGF and IGF-1 led to angiogenesis, innervation, and SC activation, resulting in functional recovery [95]. In previous research in our lab, transplantation of mesenchymal stem cells into acellular ECM increases blood vessel density, resulting in functional recovery.
CONCLUSIONSSkeletal muscles can heal in general injuries, but are not capable of regenerating across a large muscle-deficient gap. The repercussions of this severe injury induce subsequent functional deficits due to loss of nerves, vessels, and muscle mass. Moreover, people who sustain VML injury suffer from emotional distress by physical abnormalities and aesthetic deficits. The processes involved in physical and psychological rehabilitation require high medical cost burdens. Current clinical treatments for VML include the transplantation of biocompatible matrix or autologous acellular ECM. However, the transplantation of the scaffolds only has not yet achieved complete functional and morphological recovery. In contrast, SC transplantation for skeletal muscle regeneration is a well-studied medical procedure. Delivery of SCs through single myofiber has shown functional recovery and hypertrophy. RT is a well-known treatment that triggers hypertrophy and increase muscle mass, but the potential interaction between resistance training and muscle cell activation has not been studied yet. Therefore, future studies to identify novel and effective therapeutic treatments of VML injury with myofiber transplantation and/or RT are needed.
Conflict of InterestAUTHOR CONTRIBUTION Conceptualization: K Lee; Data curation: K Lee; Formal analysis: K Lee, W Park, KS Hong; Funding acquisition: K Lee, KS Hong; Method-ology: K Lee, W Park, KS Hong; Project administration: K Lee, KS Hong; Visualization: W Park, KS Hong; Writing original draft: K Lee, KS Hong; Writing-review & editing: K Lee, W Park, KS Hong. REFERENCES1. Grogan BF, Hsu JR. Skeletal Trauma Research C. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19(Suppl 1):S35-7.
2. Terada N, Takayama S, Yamada H, Seki T. Muscle repair after a trans-section injury with development of a gap: an experimental study in rats. Scand J Plast Reconstr Surg Hand Surg. 2001;35(3):233-8.
3. Friedrich JB, Katolik LI, Hanel DP. Reconstruction of soft-tissue injury associated with lower extremity fracture. J Am Acad Orthop Surg. 2011;19(2):81-90.
4. Norris BL, Kellam JF. Soft-tissue injuries associated with high-energy extremity trauma: principles of management. J Am Acad Orthop Surg. 1997;5(1):37-46.
5. Meintjes J, Yan S, Zhou L, Zheng S, Zheng M. Synthetic, biological and composite scaffolds for abdominal wall reconstruction. Expert Rev Med Devices. 2011;8(2):275-88.
6. Badylak S, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. Heart Surg Forum. 2003;6(2):E20-6.
7. Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112(9 Suppl):I144-9.
8. Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152(1):135-9.
9. Mase VJ Jr, Hsu JR, Wolf SE, Wenke JC, Baer DG, et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7):511.
10. Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637-706.
11. Brzoska E, Bello V, Darribere T, Moraczewski J. Integrin alpha3 sub-unit participates in myoblast adhesion and fusion in vitro. Differentiation. 2006;74(2-3):105-18.
12. Yu X, Dillon GP, Bellamkonda RB. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5(4):291-304.
13. Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32(14):3575-83.
14. Nandan D, Clarke EP, Ball EH, Sanwal BD. Ethyl-3,4-dihydroxyben-zoate inhibits myoblast differentiation: evidence for an essential role of collagen. J Cell Biol. 1990;110(5):1673-9.
15. McFarland DC, Doumit ME, Minshall RD. The turkey myogenic satellite cell: optimization of in vitro proliferation and differentiation. Tissue Cell. 1988;20(6):899-908.
16. Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DD. 3D timelapse analysis of muscle satellite cell motility. Stem Cells. 2009;27(10):2527-38.
17. Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30(12):2393-9.
18. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-89.
19. Park JS, Chu JS, Tsou AD, Diop R, Tang Z, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32(16):3921-30.
20. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153-62.
21. Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15(5):1119-25.
22. Mauney J, Olsen BR, Volloch V. Matrix remodeling as stem cell recruitment event: a novel in vitro model for homing of human bone marrow stromal cells to the site of injury shows crucial role of extracellular collagen matrix. Matrix Biol. 2010;29(8):657-63.
23. Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985). 2001;91(2):534-51.
24. Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, et al. In-flammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85(5):779-87.
25. Li X, McFarland DC, Velleman SG. Extracellular matrix proteoglycan decorin-mediated myogenic satellite cell responsiveness to transforming growth factor-beta1 during cell proliferation and differentiation decorin and transforming growth factor-beta1 in satellite cells. Domest Anim Endocrinol. 2008;35(3):263-73.
26. Yablonka-Reuveni Z, Seger R, Rivera AJ. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem. 1999;47(1):23-42.
27. Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Engineering Part A. 2008;14(11):1835-42.
28. Borschel GH, Dennis RG, Kuzon WM Jr. Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast Reconstr Surg. 2004;113(2):595-602 discussion 603–594..
29. Merritt EK, Cannon MV, Hammers DW, Le LN, Gokhale R, et al. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng Part A. 2010;16(9):2871-81.
30. Marzaro M, Conconi MT, Perin L, Giuliani S, Gamba P, et al. Autolo-gous satellite cell seeding improves in vivo biocompatibility of homologous muscle acellular matrix implants. Int J Mol Med. 2002;10(2):177-82.
31. De Coppi P, Bellini S, Conconi MT, Sabatti M, Simonato E, et al. Myoblast-acellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue Eng. 2006;12(7):1929-36.
32. Conconi MT, De Coppi P, Bellini S, Zara G, Sabatti M, et al. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials. 2005;26(15):2567-74.
33. Merritt EK, Hammers DW, Tierney M, Suggs LJ, Walters TJ, et al. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A. 2010;16(4):1395-405.
34. Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456(7221):502-6.
35. Schultz E. A quantitative study of the satellite cell population in postnatal mouse lumbrical muscle. Anat Rec. 1974;180(4):589-95.
36. Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec. 1982;202(3):329-37.
37. Snow MH. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anat Rec. 1983;207(4):593-604.
38. Biressi S, Rando TA. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 2010;21(8):845-54.
39. Lindstrom M, Pedrosa-Domellof F, Thornell LE. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: application to a cohort of power lifters and sedentary men. Histochem Cell Biol. 2010;134(4):371-85.
40. Rossi CA, Pozzobon M, Ditadi A, Archacka K, Gastaldello A, et al. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PLoS One. 2010;5(1):e8523.
41. Schmalbruch H, Hellhammer U. The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec. 1977;189(2):169-75.
42. Tennyson VM, Brzin M, Kremzner LT. Acetylcholinesterase activity in the myotube and muscle satellite cell of the fetal rabbit. An electron microscopic-cytochemical and biochemical study. J Histochem Cytochem. 1973;21(7):634-52.
43. Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and mus-cular system. Mech Dev. 1990;33(1):27-37.
44. Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777-86.
45. Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insu-lin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138(2):311-5.
46. Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209-38.
48. Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165(2):307-12.
49. Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, et al. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci. 1999;112(Pt 17):2895-901.
50. Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191(2):270-83.
51. Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, et al. Ki-netics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp Cell Res. 2002;281(1):39-49.
52. Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced differentiation potential of primary MyoD-/- myogenic cells derived from adult skeletal muscle. J Cell Biol. 1999;144(4):631-43.
53. Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10(10):1173-83.
54. Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD(−/-) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol. 2000;224(2):122-37.
55. Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. Identi-fication of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992;267(1):99-104.
56. Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364(6437):501-6.
57. Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, et al. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364(6437):532-5.
58. Venuti JM, Morris JH, Vivian JL, Olson EN, Klein WH. Myogenin is required for late but not early aspects of myogenesis during mouse development. J Cell Biol. 1995;128(4):563-76.
59. Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289-301.
60. Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, et al. Direct iso-lation of satellite cells for skeletal muscle regeneration. Science. 2005;309(5743):2064-7.
61. Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25(4):885-94.
62. Mouly V, Aamiri A, Bigot A, Cooper RN, Di Donna S, et al. The mi-totic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand. 2005;184(1):3-15.
63. Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120(1):11-9.
64. Boldrin L, Morgan JE. Grafting of a single donor myofibre promotes hypertrophy in dystrophic mouse muscle. PloS One. 2013;8(1):e54599.
65. Hall JK, Banks GB, Chamberlain JS, Olwin BB. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci Transl Med. 2010;2(57):57ra83.
66. Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol (1985). 2002;93(4):1318-26.
67. MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interi-sano SA, et al. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20(4):480-6.
68. Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145-68.
69. Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34(10):663-79.
70. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985). 2008;104(6):1736-42.
71. Thornell LE, Lindstrom M, Renault V, Mouly V, Butler-Browne GS. Satellite cells and training in the elderly. Scand J Med Sci Sports. 2003;13(1):48-55.
72. Chen XK, Walters TJ. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. J Plast Reconstr Aesthet Surg. 2013;66(12):1750-8.
73. Corona BT, Wu X, Ward CL, McDaniel JS, Rathbone CR, et al. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials. 2013;34(13):3324-35.
74. Herdy AH, Marcchi PL, Vila A, Tavares C, Collaco J, et al. Pre- and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: a randomized controlled trial. Am J Phys Med Rehabil. 2008;87(9):714-9.
75. Lucia A, De La Rosa A, Silvan MA, Lopez-Mojares LM, Boraita A, et al. Mobilisation of mesenchymal cells in cardiac patients: is intense exercise necessary? Br J Sports Med. 2009;43(3):221-3.
76. Green H, Goreham C, Ouyang J, Ball-Burnett M, Ranney D. Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am J Physiol. 1999;276(2):R591-6.
77. Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with inter-val sprint training versus aerobic endurance training. J Physiol Phar-macol. 2008;59(Suppl 7):71-88.
78. Teixeira CF, Zamuner SR, Zuliani JP, Fernandes CM, Cruz-Hofling MA, et al. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with bothrops asper snake venom. Muscle Nerve. 2003;28(4):449-59.
79. Borisov AB, Dedkov EI, Carlson BM. Abortive myogenesis in denervated skeletal muscle: differentiative properties of satellite cells, their migration, and block of terminal differentiation. Anat Embryol (Berl). 2005;209(4):269-79.
80. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-64.
81. Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, et al. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Com-mun. 2007;359(4):915-20.
82. Son YJ, Thompson WJ. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995;14(1):133-41.
83. Kopp DM, Trachtenberg JT, Thompson WJ. Glial growth factor res-cues Schwann cells of mechanoreceptors from denervation-induced apoptosis. J Neurosci. 1997;17(17):6697-706.
84. Trachtenberg JT, Thompson WJ. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379(6561):174-7.
85. Jacobson C, Duggan D, Fischbach G. Neuregulin induces the expression of transcription factors and myosin heavy chains typical of muscle spindles in cultured human muscle. Proc Natl Acad Sci USA. 2004;101(33):12218-23.
86. Ruberti F, Capsoni S, Comparini A, Di Daniel E, Franzot J, et al. Phenotypic knockout of nerve growth factor in adult transgenic mice re-veals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J Neurosci. 2000;20(7):2589-601.
87. Lavasani M, Lu A, Peng H, Cummins J, Huard J. Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle. Hum Gene Ther. 2006;17(2):180-92.
88. Taylor MD, Vancura R, Patterson CL, Williams JM, Riekhof JT, et al. Postnatal regulation of limb proprioception by muscle-derived neuro-trophin-3. J Comp Neurol. 2001;432(2):244-58.
89. Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374(6521):450-3.
90. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677-736.
92. Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamy-cin (mTOR)-dependent signaling. FASEB J. 2002;16(8):771-80.
93. Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, et al. Histone deacety-lases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7(4):437-43.
|
|
|||||||||||||||||||||||||||||||||||||||