Human Epidermal Growth Factor Improves Atopic Disease-like Skin Lesions in DFE/DNCB Induced BALB/c Mice and Human Keratinocytes
Article information
Trans Abstract
PURPOSE
Atopic skin disease is an inflammatory disease with a multifactorial pathogenesis that presents as itchy, dry skin. Human epidermal growth factor (EGF) plays an essential role in regulating cell growth, proliferation, and differentiation. Accordingly, this study aimed to investigate the effects of EGF on atopic diseases.
METHODS
Atopic disease was induced by the Dermatophagoides farinae house dust mite extract and 2,4-dinitrochlorobenzene in BALB/c mice. The mice were divided into the following five atopic disease induction and treatment groups: control, atopic disease only, atopic disease+epidermal growth factor-1, atopic disease+epidermal growth factor-10, and positive control atopic disease+ceramide. We examined dermal and epidermal ear thickness, mast cell and T cell infiltration, serum immunoglobulin E and immunoglobulin G2a levels, and mRNA expression of pathogenic cytokines in murine ear tissue and human keratinocyte cells.
RESULTS
Epidermal growth factor treatment increased epidermal and dermal ear thickness and inhibited mast and T cell infiltration in the ear in a dose-dependent manner. Treatment also suppressed immunoglobulin E and G2a levels and the mRNA expression of pathogenic cytokines in murine ear tissue and human keratinocytes. The therapeutic outcome of epidermal growth factor was stronger than that of the positive control ceramide.
CONCLUSIONS
EGF may be beneficial in the treatment of atopic disease.
INTRODUCTION
Atopic disease (AD) is a well-known inflammatory allergic disease that starts with itchy, dry skin [1]. Although AD usually begins at an ear-ly age or in childhood, first episodes in middle age are also possible, with the potential for AD to persist throughout life. AD has a worldwide inci-dence of 2-10% among adults and 15-30% among children [2]. The etiol-ogy of AD is multifactorial in nature, with several hypotheses regarding its pathogenesis having been proposed, including impaired immune response, genetics, skin barrier dysfunction, and environmental factors [3–5]. AD is characterized mainly by T cell polarization. In the acute form of the disease, T helper 2 (Th2) cell-mediated lesions and inflammatory cytokines, including interleukins (ILs) IL-6, IL-17, and IL-13, have been reported, while the chronic form of the disease is associated with the Th1 phenotype [6,7]. Furthermore, tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) are very important factors [8,9]. Thymic stromal lymphopoietin (TSLP) is a skin epithelial-derived cytokine, with its irregular function has also been associated with AD [10,11].
In its mild form, AD is not very itchy and usually recovers with the use of moisturizers. However, in its severe form, AD affects a large area of skin, is very itchy, and does not recover with the use of moisturizers. Generally, topical steroids and moisturizers, which preserve moisture in the dermal membrane and reduce inflammation, are used to treat AD, although steroids are associated with minor side effects [12]. Growth factors are proteins secreted by all cell types, including keratinocytes which constitute the dermis and epidermis of the skin. The biology of these factors differs slightly from that of usual hormones, based on their site of synthesis and action [13]. Epidermal growth factor (EGF) up-regulates the proliferation, growth, and differentiation of cells [14,15]. Alexander et al. [16] showed that the EGF promotes mammalian cell growth factor by reducing cellular senescence (biological aging). The topical use of EGF was found to accelerate wound healing in rats [17]. However, the effect of EGF on AD, with regards the immune system, has not yet been studied.
Our aim in this study was to evaluate the inhibitory effects of human EGF on AD by inducing AD using house dust mite (Dermatophagoides farina) extract (DFE) and 2,4-dinitrochlorobenzene (DNCB) in BALB/c mice. We examined dermal and epidermal ear thickness, T cell infiltration and mast cell, serum immunoglobulin (Ig) E and IgG2a levels, and mRNA gene expression of pathogenic cytokines in the mouse ear tissue. Effects were also evaluated in human keratinocyte cells.
METHODS
1. Materials
Human keratinocyte (HaCaT) cell lines were purchased from ATCC (Manassas, VA, USA). DNCB and mite extracts were obtained from Sig-ma–Aldrich (St. Louis, MO, USA). RNA isolation of TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). The mouse IgE and IgG2a ELISA kits were obtained via BD Biosciences (San Jose, CA, USA).
2. Procedure for human EGF
Human epidermal growth factor (hEGF) was produced by introducing and expressing a gene encoding the same protein as hEGF into Escherichia coli and then separating and purifying it to high purity using protein sepa-ration and purification technology (Bioprogen, Daejeon, South Korea).
3. Animals
Seven-week-old female BALB/c 40 mice were obtained from Samtako (Osan, Republic of Korea) and were kept under specific pathogen-free conditions. Mice were divided into five groups at 8 animals. All experi-ments were approved by the Animal Care Institutional and Use Com-mittee of the Konkuk University.
4. Induction of atopic lesions in the ear and blood analysis
AD was induced in mice by repeated local exposure of the ear skin to DFE/DNCB, as previously described [18]. For the induction of AD, mice were divided into five groups, as follows: control, AD-only, AD+EGF1 ppm/ear, AD+EGF10 ppm/ear, and AD+ceramide 15ug/ear [CERA]. The surfaces of both earlobes were stripped 4–5 times using 3.5 cm tape (Sin-sin, Korea). After stripping, 10 µL of one percent DNCB was painted on each ear, followed by 10 µL of DFE (10 mg/mL) 4-5 days later. DFE or DNCB was painted alternately once per day for 35 days. The animals re-ceived a positive control CERA throughout the 35 days of AD induction. Ear thickness was measured 20 hours after application of DNCB or DFE using a dial thickness (Kori Seiki MFG, Co., Japan). On day 35 post-in-duction, blood samples were collected by heart puncture. Plasma samples were prepared from the blood samples and stored in a deep freezer. After blood sample collection, the ear tissue was removed and histopathological analysis was performed. Serum IgG2a and immunoglobulin (Ig)E levels were also measured using an immunoassay kit (Bethyl Laboratories, Inc., Montgomery, TX, USA), as reported by the manufacturer's instructions.
5. Ear tissue histological observations
The excised ear tissue was fixed in ten percent paraformaldehyde for 20 h and embedded in paraffin. Thin, 7-µm, sections were stained using hematoxylin and eosin (H&E). The ear tissue thickness of the dermis and epidermis were measured using a microscope. Both T cells and mast cells play critical roles in the development of AD and are useful markers. To measure mast cell and T cell infiltration, dermal sections were stained with toluidine blue and the mast cell number was counted in six randomly chosen fields of view.
6. Real-time polymerase chain reaction measure
Quantitative real-time polymerase chain reaction measure (PCR) was performed using Thermal Cycler Dice Gradient TP8500 (Takara Bio Inc., Shiga, Japan) according to the manufacturer's method. Total RNA extraction was performed from the ear tissues for each group. The real-time PCR was performed as previously described [18]. Briefly, 2 μL of cDNA sample (100 ng), 2 μL of antisense and sense primer solution (0.5 µM), 10 μL of Ex Taq SYBR Premix (Takara Bio Inc.), and 10 μL of dH2 O were mixed to obtain a total volume of 24 μL in each reaction tube.
The mouse primers design used for real-time PCR were as follows: mouse TNF-α, sense′-AAG CCT GTA GCC CAC GTC GTA- antisense′ and sense′-GGC ACC ACT AGT TGG TTG TCT TTG- antisense′; mouse IFN-γ, sense′-TCA AGT GGC ATA GAT GTG GAA GAA- anti-sense′, and sense′-TGG CTC TGC AGG ATT TTC ATG- antisense′; mouse IL-13, sense′-CCT ACC AGA CCA AGG TCA AC- antisense′ and sense′-AGG GGG TAA TAA AGG GAT TG- antisense′; mouse GAPDH, sense′-GCA CAG TCA AGG CCG AGA AT- antisense′, and sense′-GCC TTC TCC ATG GTG GTG AA- antisense′. Human TNF-α, sense′-CCA CCA GAC CAA GGT CAA C-antisense′, sense′-AGG GGG TAA TAA AGG GAT TG- antisense′ human IL-6, sense′-AAA GAG GCA CTG CCA AAA A- antisense′ sense′ human IL-17 sense′-TCC CCT CTG TCA TCT GGG AAG- antisense′ sense′-CTC GAC CCT GAA AGT GAA GG- antisense. The amplification conditions were as follows: 10 seconds at 94°C, 35 cycles of 6 seconds at 94°C and 25 seconds at 59°C, 15 seconds at 94°C, 25 seconds at 59°C, and 13 seconds at 94°C. The mRNA expression levels for target genes relative to GAPDH were normalized using the following formula: relative mRNA gene expression=2− (ΔCt of target gene − ΔCt of GAPDH), where the Ct value is the threshold measure of cycle value. The mRNA gene expression levels of the target genes relative to the housekeeping gene GAPDH were normalized, as follows: in each sample, the gene expression level of the explored gene was normalized to the level of GAPDH and measured as a relative mRNA gene expression level.
7. Cell culture
Human keratinocyte HaCaT cells were cultured in Gibco Dulbecco's modified Eagle's medium added to ten percent Gibco fetal bovine serum and 10 µg/100 mL penicillin and streptomycin in a five percent CO2 en-vironment at 37°C. For the assay, HaCaT keratinocyte were pre-treated for IFN-γ (10 ng/mL) and TNF-α (10 ng/mL). After 5 hours of incubation, cells were harvested, and total protein and RNA were isolated.
8. Statistical analysis
Statistical analysis was performed using the SAS statistical software program (SAS Institute, Cary, NC, USA). Between-group differences were evaluated using a one-way analysis of variance, followed by Dun-nett's test for multiple comparisons. All data were expressed as the mean ±standard deviation (SD) of the comparative fold difference. The threshold value for significance was set at p <.05.
RESULTS
1. Partial chemical characterization of EGF and induction of AD in the mouse ear
The therapeutic effects of EGF on AD induced in BALB/c mice were evaluated by alternately painting DFE and one percent DNCB on both earlobes for 35 days post-induction (Fig. 1A). Recombination of EGF in the Bioprogen was confirmed by SDS-PAGE (Fig. 1B). Ear tissue thickness and AD were increased by DFE/DNCB. The ear thickness and mast and T cell infiltrations in ear tissue were examined after topical treatment with EGF. EGF application reduced ear thickness and AD compared to in the AD group (Fig. 1C). EGF treatment decreased the number of lesions on the ears at day 35 after AD induction from five in each group (Fig. 1D).
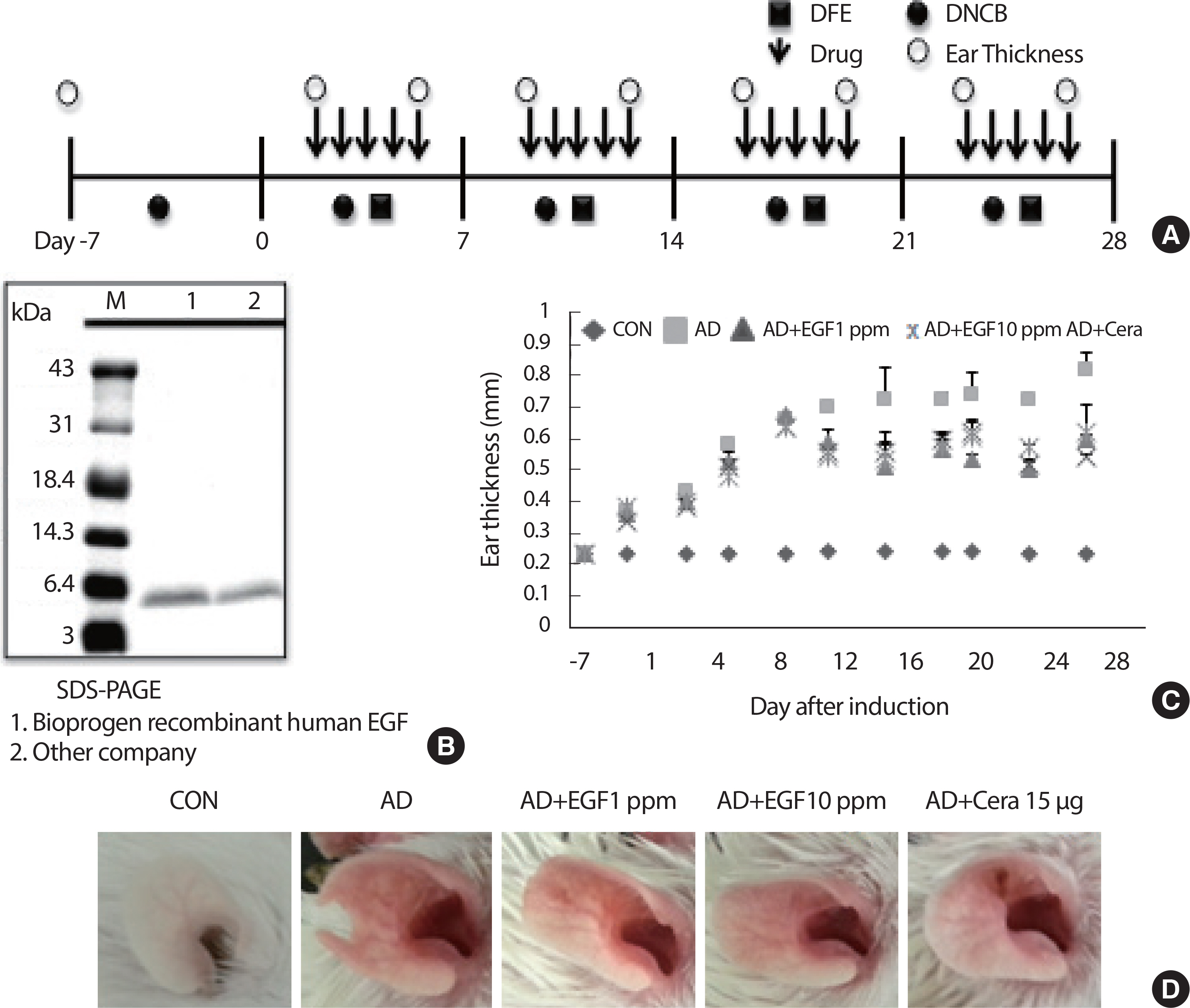
EGF treated attenuates the manifestations of AD in mice. (A) Schematic depiction of the induction and EGF treatment of atopic dermatitis. (B) SDS-PAGE of EGF used in this study. (C) Ear thickness was measured with a dial thickness gauge every three days after DNCB/DFE application. (D) Representative photographs of mouse ears for each group at 35 days after AD induction are shown. DFE, Dermatophagoides farina extract; DNCB, 2, 4-dinitrochloro-benzene; EGF, epidermal growth factor; CON, control; CERA, ceramide; AD, atopic disease.
2. EGF reduces mouse ear tissue inflammation and immune cell infiltration
To determine whether EGF painting affects DFE/DNCB-induced im-mune-cell infiltration and inflammation in the ear tissues, tissue histological analysis was performed. H&E stained mouse ear tissue sections revealed typical pathological characteristics in the AD mice group, namely parakeratosis and hyperkeratosis. EGF application reduced ear tissue staining and AD lesions compared to the AD control group (Fig. 2A). Toluidine blue staining and TUNEL assays of the ear tissue sections reduced the presence of mast cells, whereas immunofluorescent staining for CD4+ indicated T cell infiltration. The reduction in tissue infiltration with EGF, compared to the AD control group, was dose-dependent (Fig. 2A, B). In the AD control group, thickening of the dermis and epidermis was observed; by comparison, dermal and epidermal ear thickening was significantly lower in EGF Group, with this decrease being dose-dependent (Fig. 2C, D). Moreover, in the EGF treatment group, there were significantly fewer T cells and mast cells in the ear tissue compared to the AD control group (Fig. 2E, F), as well as a reduction in IgE and IgG2a levels, with these decreases being concentration-depen-dent (Fig. 2G, H).
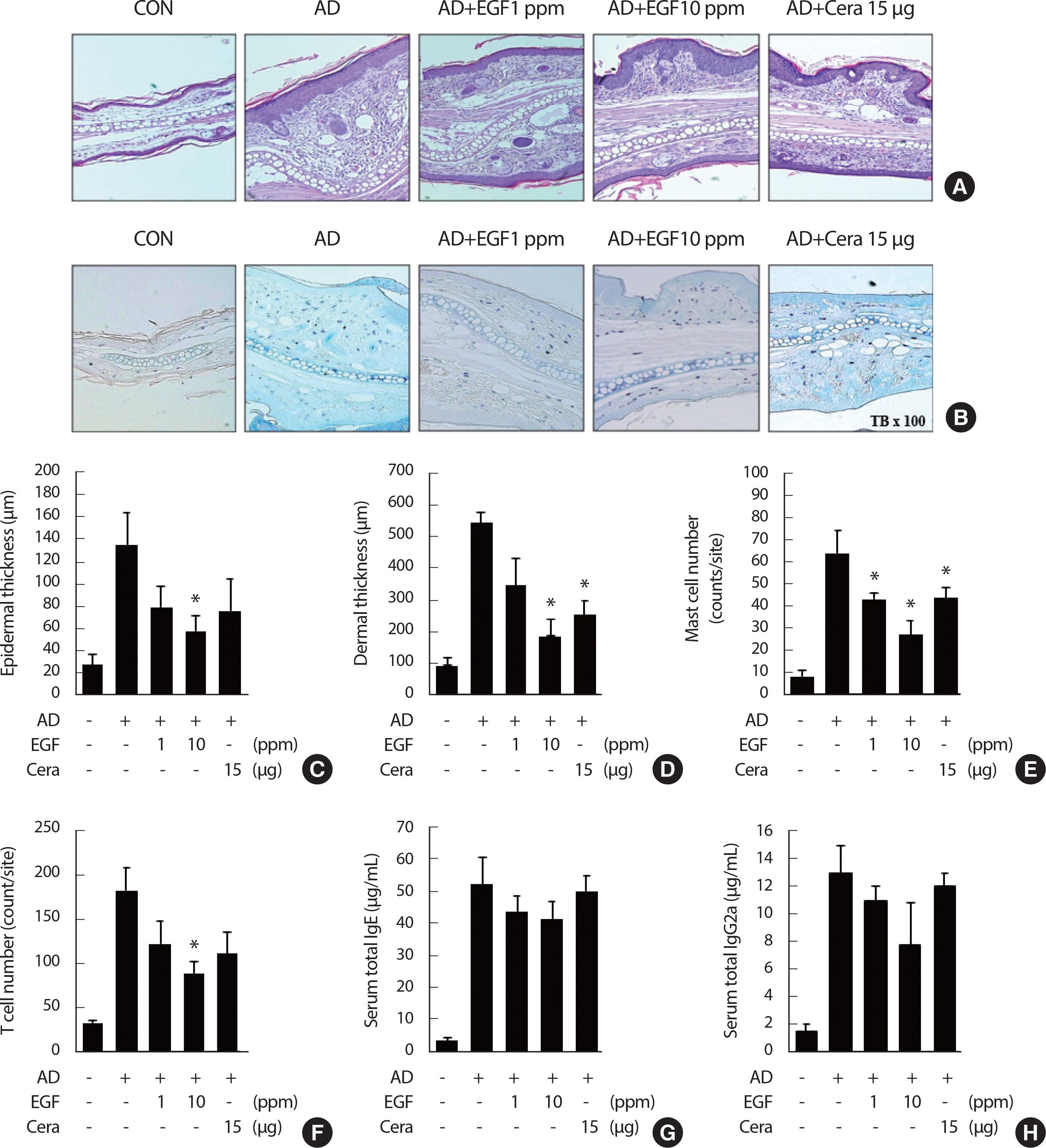
Treatment of EGF reduces tissue inflammation and immune cell infiltration in AD. (A and B) Microphotographs of tissue sections of the right ear at four weeks after AD induction. The tissue sections were stained using hematoxylin and eosin (H&E), toluidine blue staining (A and B). The original magnifi-cation was X 100. (C and D) Epidermal thickness and dermal thickness was measured from H&E stained (A) microphotographs. (E and F) The cell number of infiltrating mast cells (E) and CD4+ T cells staining in the right ear sections, as revealed by toluidine blue and anti-CD4 antibody staining (F), respectively. At least five randomly chosen sites were selected for each cell number count analysis. (G and H) The total IgE level (G) and total IgG2a levels (H) in the sera from the five mouse groups were measured by enzyme-linked immunosorbent analysis. Mice blood samples were collected by cardiac puncture at four weeks after AD induction. The data are presented as the mean±SD (n = 6/group). EGF, epidermal growth factor; CON, control; CERA, ceramide; AD, atopic disease. * p<.05 compared to the AD control group.
3. EGF treatment downregulates the gene expression of immunoregulatory cytokines in the ear tissue of AD mice
Tissue inflammation induced in AD mice is secondary to enhanced effector regulatory T cell infiltration functions, such as the proinflammatory cytokines production [19]. Using real-time PCR, EGF treatment significantly lower mRNA gene expression of Th1 type cytokines IFNγ and TNFα, compared to the AD control. EGF treatment also significantly decreased the expression of Th2-type cytokines IL-6 and IL-13. The expression of IL-17, an immunoregulatory cytokine released by Th17 cells, was also downregulated in the EGF treated group, compared to the AD control group. Similarly, TSLP, a keratinocyte-derived Th2-type cytokine that drives inflammatory responses, was also downregulated by EGF, in a dose-dependent manner (Fig. 3).
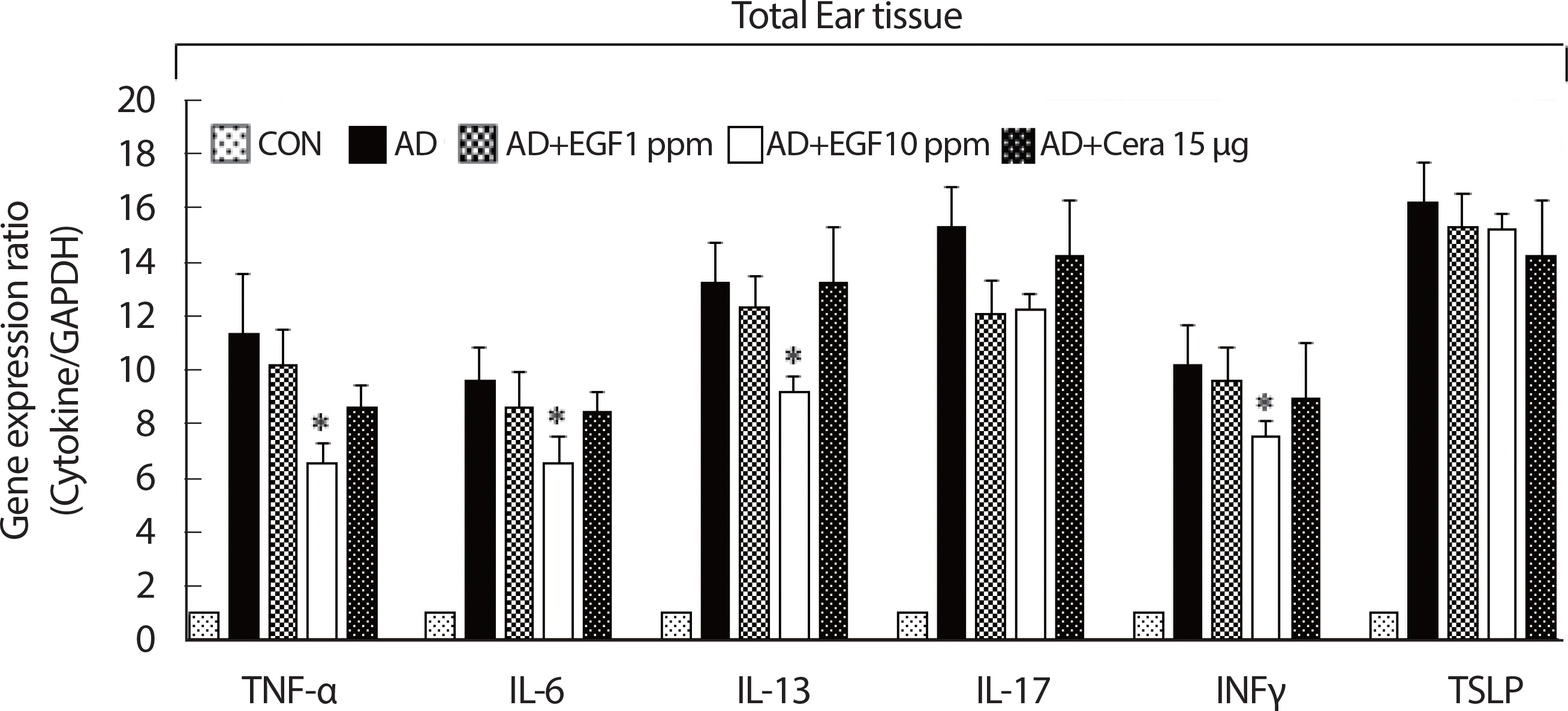
The effect of EGF on the expression of various pathogenic cytokine-related mRNA in control or AD-induced mouse ear tissue. The ears were excised on four weeks and total mRNA was isolated. Quantitative real-time polymerase chain reaction was performed as described in the Methods. Data are presented as the mean±SD of triplicate determinations. AD, atopic dermatitis; EGF, epidermal growth factor; CON, control; CERA, ceramide. *significant difference compared to the value for the DFE/DNCB-treated mice (p<.05).
4. Inhibitory effect of hEGF on inflammatory stress in stimulated keratinocytes
The ability of EGF to inhibit the effects of proinflammatory cytokine mediators in HaCaT cells was assessed using RT-PCR, with its inhibitory effect on proinflammatory mediators cytokine TNF-α/IFNγ (10 ng/mL) in HaCaT cells assessed using RT-PCR analysis. Human EGF extract reduced the mRNA gene expression levels of IL-6, IL-17, and TNF-α following TNFα/IFNγ induction in a dose-dependent manner (1, 10 ppm) (Fig. 4A), indicating that EGF extract inhibited proinflammatory cytokine and chemoattractant production.
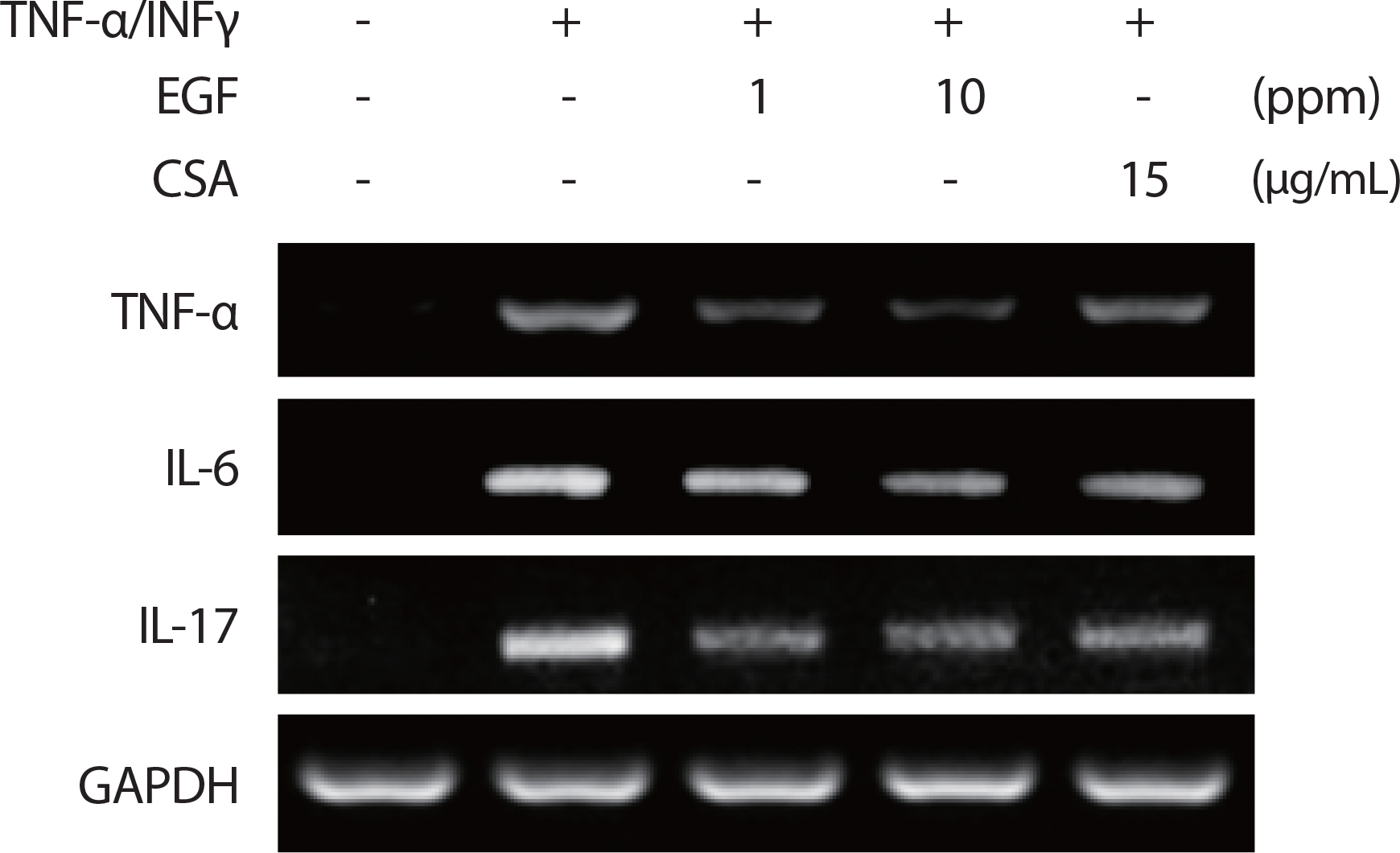
Treatment of EGF reduces the production of proinflammatory cytokines. HaCaT cells were pre-incubated for 30 minutes after EGF application with TNF-α/IFN-γ (10 ng/mL) and CSA (15 ug/ml). After 5 hours of stimulation, cells were harvested and total mRNA was isolated. Gene expression levels of proinflammatory cytokines TNFα, IL-6, and IL-17 were measured by conventional RT-PCR. TNF, tumor necrosis factor; EGF, epidermal growth factor; IL, interleukin; CSA, cyclosporin.
DISCUSSION
Previous studies have aimed at improving a topical formulation for the persistent and established pharmacological properties of EGF [19,20]. A recent study reported that a recombinant human origin EGF-based cream prevented radiation-induced dermal disease in patients with breast cancer [21]. In our study, the therapeutic effects of EGF on AD were evaluated using AD induced in BALB/c mice through the application of DFE and DNCB for four weeks (Fig. 1A). Ear tissue thickness and AD were increased by DFE/DNCB, with reduction in ear thickness and mast and T cell infiltrations in ear tissue after topical treatment with EGF (Fig. 1C-D). Mast cells plays an important role in inducing inflammation, with inflammatory mediators, such as histamine and cytokines, released from stimulated mast cells. Therefore, histological analysis of ear tissue after AD induction was performed to determine the optimal evaluation of AD symptoms. As expected, EGF application significantly decreased mast cell number and T cells infiltration, in a dose-dependent manner, compared to the AD control group (Fig. 2A, B). Interestingly, EGF10 showed higher activity than the positive control CERA. Additionally, EGF treatment suppressed epidermal and dermal thickening (Fig. 2C, D).
A high IgE level is an important sign of AD progression [22]. AD is a typical Th2 type immune disorder that results in the upregulation of serum IgE level and Th2 type cytokine gene expression [23,24]. In addition, serum IgG2a production is linked to the Th1 type response [25]. Furthermore, higher Th1 type cytokines, such as IFN-γ, are important in the pathogenesis and management of AD [23]. To determine whether EGF exerts its effects mainly via the Th1 and Th2 response, IgG2a and IgE serum levels were examined in each treatment group. AD mice had elevated IgG2a and IgE levels compared to the control mice. The mice treated with EGF showed significantly reduced IgG2a and IgE levels, in a concentration-dependent manner (Fig. 2G, H). The therapeutic effect of EGF was more effective compared to the positive control CERA.
To determine the Th1 or Th2 responses and the underlying mecha-nism of EGF, we verified the mRNA gene expression levels of AD-relat-ed pathogenic cytokines of mice ear tissues using real-time PCR. In AD mice, all cytokine-related mRNA levels were increased. Topical application of EGF significantly suppressed the expression of both Th1 type and Th2 type cytokines, such as Th1-related cytokines IFN-γ and TNF-α, and Th2-related cytokines IL-6, IL-13, and IL-17 in the mice ear tissue (Fig. 3).
The effects of EGF10 were more robust than those of CERA. TSLP is closely related to IL-7 cytokines and plays an important role in allergic diseases, including AD [26]. Therefore, we examined the gene expression of TSLP in the ear tissue and found that EGF suppressed the level of TSLP in a dose-dependent manner (Fig. 3). In addition, high levels of TSLP were found in the dermal cells of patients with AD but not in those with unaffected skin [27].
Many researchers have examined the biological effects of cytokines on human keratinocyte cells and reported that keratinocytes are markers for a specific set of inflammatory cytokines in inflamed skin [28]. The IL family has been shown to be an important regulator of epidermal function and their receptors have been observed in keratinocytes [29]. In the present study, the effect of EGF on human keratinocytes was induced by the proinflammatory cytokine mediators TNF-α and IFN-γ (10 ng/ml), as assessed by RT-PCR (Fig. 4). EGF reduced IL-6, IL-17, and TNF-α mRNA gene expression in TNF-α/IFN-γ-induced cells in a dose-dependent manner. This finding indicates that EGF can reduce the production of proinflammatory cytokines in human keratinocytes. Furthermore, elevated cytokine levels in the affected skin have been shown to cooperate to induce TSLP production by keratinocytes, suggesting an inflammatory pathway [30], consistent with our findings.
To summarize, we examined the anti-AD effect of a topical administration of EGF using an animal AD model. Topical use of EGF effective-ly downregulated the severity of AD, such as histopathological symptoms, production of IgE, IgG2a, and gene expression of pathogenic cyto-kine-related mRNA in DFE/DNCB-induced BALB/c mice and human keratinocytes. In conclusion, our study suggests that EGF actively sup-presses AD lesions by controlling the inflammatory response.
Notes
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Conceptualization: NK Kim, SH Kim, BH Park, and EJ Choi; Data curation: NK Kim, SH Kim, BH Park, and EJ Choi; Formal analysis: NK Kim, SH Kim, BH Park, and EJ Choi; Funding acquisition: NK Kim, SH Kim, BH Park, and EJ Choi; Methodology: NK Kim, SH Kim, BH Park, and EJ Choi; Project administration: NK Kim, SH Kim, BH Park, and EJ Choi; Visualization: NK Kim, SH Kim, BH Park, and EJ Choi; Writing - original draft: NK Kim, SH Kim, BH Park, and EJ Choi; Writing - re-view & editing: NK Kim, SH Kim, BH Park, and EJ Choi.
