Neurovascular Interaction and Exercise Training for Bone Regeneration
Article information
Abstract
The nervous and vascular systems are widely distributed in the skeletal system and play an important role in bone metabolism and bone formation, respectively. Their independent impact on the skeletal system has received keen attention in bone-related research over the decade. However, the mechanism of neurovascular coupling during physiological bone remodeling and regeneration has recently been highlighted, considering the importance of spatial relationships between bone-associated skeletal nerves and blood vessels. In addition, the positive effect of exercise on the bones has been continuously emphasized, as evident by the improved number and function of skeletal nerves and blood vessels following exercise training. Skeletal nerve-vascular crosstalk and exercise training are essential for bone development and regeneration, respectively; however, the effect of exercise on neurovascular interactions has not yet been studied. This review aims to summarize the regulatory roles of the nerves and blood vessels in bone metabolism and regeneration and to highlight a combination of potential cellular processes of neurogenesis and angiogenesis in bone regeneration. Moreover, given the significance of the spatial relationship between nerves and blood vessels in bones and the role of exercise training, this review aims to discuss the potential physiological neurovascular coupling that occurs following exercise and physical activity.
INTRODUCTION
Investigations on the skeletal nervous system have been ongoing for decades, as the nervous system is imperative for the health and longevity of all biological tissues and organisms. However, this topic is becoming a hotspot. Recently, the regulatory roles of the skeletal nervous system were thoroughly addressed, including the sensory, sympathetic, and parasym-pathetic nerve actions innervating the bone tissue [1-6]. These research studies have revealed that each subtype of nerve fibers has distinctive functions within the bone microstructure and metabolism [7-9]. Besides providing the general roles of nerve fiber, such as the motor control to the muscle fibers, delivering sensory information from the periphery to the central nervous system, and regulates the autonomic functions, skeletal nerve fibers stimulates neural signals [1-6]. The growth of the peripheral nerve fibers, the initial innervation, and the neurotransmission into the bone tissue regulate bone remodeling and metabolism. Furthermore, nerve ingrowth is an essential upstream mediator for the endochondral ossification process during bone growth [10] and intramembranous bone formation [5,6]. Thus, the sensory and pain-related sensations and nerve stimuli are a constant necessity for the healthy maintenance of the skeleton.
Besides nerve stimulation for bone metabolism, bone blood vessels are indispensable for skeletal health by providing oxygen and essential nutrients and delivering systemic hormones and precursor cells of osteoblasts and osteoclasts [11]. Moreover, they participate in hematopoiesis in the bone marrow region [12]. Several reports suggest that modification of bone blood vessels by the stimulating factors occurs more promptly than the alteration in bone [13-16], and suppressing the vascular responses to the stimuli causes a decline in vascular function and blood flow, and angiogenesis diminishes bone metabolism and bone gain [14,16-18].
Recent research demonstrated that exercise training or physical activity modifies peripheral nerve activity, bone vascular function, and angiogenesis. Based on the previous literature, changes in the bone-associated skeletal nerve density [19], peripheral nervous system [20-22] and vascular system [13,16,18] through exercise are likely to have a positive effect on bone metabolism. However, in the last couple of years, the topic of bone-associated skeletal nerve and bone blood vessel interaction has been emerging, and its potential role in bone metabolism and regeneration has been highlighted. Despite the attention from these independent observations, the extent to which exercise-induced increases in neurovascular interaction contributes to improvements in skeletal remodeling and metabolism remains an intriguing unanswered question. In this review, we cover the potential roles of neurovascular coupling in bone formation and regeneration and the regulation of the neurovascular system by exercise training, and provide an overview of the prospective mechanisms of mutual adaptation between the neurovascular system and bone.
CURRENT UNDERSTANDING OF NEUROVASCULAR INTERACTION IN BONE PHYSIOLOGY
The relationship between bone and nerves has been widely studied. The periosteum of the bone is covered primarily by sensory and sympathetic axons [23] that play a crucial role in regulating bone formation and regeneration [5,24]. Murine injury model studies have discovered that sensory denervation by surgical and chemical methods impaired bone fracture or compromised the repair process and blunted bone formation [25-28]. The regulatory role of sensory nerves in bone formation has been observed in mammalian models and human patients with neurological dysfunction. Cao et al. reported that loss of the sensory nerves reduced the new bone quality in rabbits [29]. Moreover, reduced peripheral innervation was associated with fracture healing [30]. A crosstalk between bone and nerves activates and regulates signaling pathways affecting bone growth and innervation. The relationship between bone-associated skeletal nerves and cells involved in bone formation has not been fully identified, but neurotransmitters derived from peripheral nerves are known to affect osteoblastogenesis [31,32]. In addition, osteoblasts are regulated by norepinephrine released from peripheral nerves [33,34], and bone permits nerves to inhabit the bone microenvironment [35]. Recently, spatial transcriptomics of the developing cranium revealed the importance of nerve signaling in regulating bone patterning and differentiation of bone precursors, highlighting the important role of neuronal signaling in tissue regulation [36].
Indispensable roles of the vascular system for bone and bone marrow function have been suggested in several ways. The demand for oxygen, nutrients, hormones, and waste product elimination requires the development of a vascular network during bone remodeling and regeneration. Additionally, the bone marrow produces immune cells [37] and precursor cells involved in bone remodeling. As one of the essential elements of the bone multicellular unit and bone remodeling compartment, blood vessels primarily transport the immune and precursor cells to and from bone and bone marrow [38-40]. Furthermore, composing the hematopoietic stem cell niches in the bone marrow to maintain bone homeostasis is one of the essential roles of bone blood vessels [41]. As with bone-associated skeletal nerves, bone vascular dysfunction is associated with reduced bone metabolism and decreased bone marrow function [42-44]. For example, declines in bone blood flow and vasodilator function are associated with diminished bone mass and bone metabolism [13,17,45] both in rodents and humans. During bone healing, inflamma-tory action begins following bone defect or fracture, leading to fibrovascular regeneration and inflow of mesenchymal stem cells (MSC) into the defect area, accelerating treatment [46].
The concept of neurovascular coupling in bone-associated skeletal nerves emerged in the 1980s after reports of increased blood flow in the joints of patients with diabetic neuropathy [47]. After that, several studies have revealed the anatomical relevance between nervous and vascular systems. For example, bone marrow blood vessels are surrounded by tyrosine hydroxylase (TH) positive sympathetic nerves [48]. In the brain, neuropeptide Y (NPY) fibers are observed around cerebral blood vessels [49] and in the periphery of large amounts of blood vessels in the medul-lary cavity of the tibia and femur [9]. Similarly, in a murine model, TH+ sympathetic nerves and calcitonin gene-related peptide (CGRP)+ sensory nerves are co-localized with platelet endothelial cell adhesion molecule (CD31)+ blood vessels in the periosteum [50]. In our prior observations, CD31 immunostaining was parallel and close to Thy1-YFP pan-neuro-nal reporters during calvarial defect healing [6]. In addition, nerve fibers are closely associated with blood vessels during skeletal development and bone repair processes [5,10].
FACTORS INVOLVED IN NEUROVASCULAR COUPLING IN BONE REMODELING
In prior work, increased neuronal ingrowth and augmented bone-as-sociated skeletal nerve density accompanying positive changes in vascular density were observed during bone development, bone defect healing, and fracture repair [5,6,10]. Similarly, heterotopic ossification, a disease that results from abnormal osteochondral differentiation of MSC and causes abnormal ossification process in the soft tissue, also showed the importance of neurovascular coupling for aberrant endochondral ossification process [51]. Spatial neurovascular resemblance during bone formation is currently considered a well-established phenomenon, but the distinct mechanisms defining the signaling pathways or involving factors between nerves and blood vessels in bone regeneration have re-mained equivocal. Earlier research using a transcriptional approach has demonstrated the regulatory relationship between bone-associated skeletal nerve signaling and bone blood vessel signaling. In the broad view emerging from these the analyses, vascular-derived neurogenic factors and neural-derived angiogenic factors facilitate neurovascular signaling in the regulation of bone metabolism and regeneration.
1. Transcriptional changes of neurogenic marker among vascular cell clusters
Various types of nerve fibers are observed in the bones, but most nerves are composed of sensory nerves that express Tropomyosin receptor kinase A (TrkA), which is required for the proliferation of osteochondral progenitor cells during the endochondral ossification process [48,52]. It was reported that nerve regeneration occurs before revascularization in the process of bone defect healing or fracture repair, and nerve regeneration contributes to blood vessel regeneration [5,6]. Since it is unknown which secreted factors are involved in the regeneration of bone-associated skeletal nerve fibers and further bone regeneration, it seems necessary to identify the neurogenic factors from bone blood vessels involved in the process. Thus, the transcriptional changes in neurogenic factors expressed by blood vessel-related cell clusters were first identified. Previously, the single-cell RNA sequencing (scRNA-Seq) profiling during endochondral ossification was examined. Cells were identified as endothelial cell (Marker gene: Pecam1, Cdh5), pericyte (Pdgfrb, Acta2), smooth muscle cell (Myh11, Abcc9, Notch3, Mcam), and lymphatic endothelial cell (Lyve1, Prox1, Flt4) clusters. Among the 45 potential neurogenic marker genes, 40 genes were identified with scRNA-Seq, and among 40 genes, 15 were highly expressed and/or upregulated over time in the vascular cell cluster.
Among these 15 genes, a gene for a neurotrophin, nerve growth factor (Ngf), was strongly expressed in the smooth muscle cell cluster, and genes for axonal guidance molecules semaphorin3a (sema3a) and semaphorin3f (sema3f) were strongly expressed in the lymphatic endothelial cell cluster (Figure 1). Nerve growth factor (NGF) is the major neurotrophic factor that regulates nerve growth, neuron survival, and regeneration [53]. Furthermore, NGF directly controls its high-affinity receptor TrkA+ sensory nerve fibers and determines the amount of innervation and growth of the sensory nerve fiber to the tissue that needs nerve stimulation or signaling [54]. The NGF-induced TrkA+ sensory innervation into the long bone cartilage membrane (growth plate) facilitates osteogenic progenitor cell differentiation and bone mineralization [10,55]. Semaphorin 3A and 3F are renowned axon-guidance molecules essential for the general development of peripheral neurons that allow nerve growth and regeneration [56]. These vascular cell cluster-derived neurogenic factors also control bone remodeling by regulating sensory innervation [57]. Surprisingly, the role in bone formation played by and is independent of the bone formation cell, the osteoblast. While the deletion of reduced bone mass, the specific deletions of from osteoblasts did not change bone mass [57]. This suggests that the role of in bone formation is to regulate sensory nerves, not through direct osteoblast activity.

Relative expression of neurogenic genes among vascular cell clusters. (A) Representative UMAP conception of three vascular cell clusters including endothelial cell, smooth muscle cell, and lymphatic endothelial cell among 17 different cell clusters. Among the 45 potential neurogenic marker genes, 40 genes were analyzed by single-Cell RNA-Seq. Among 40 genes, 15 are highly expressed and/or upregulated over time in the vascular cell clusters. (B) Violin plots showing overexpressed neurogenic gene markers including Ngf, Sema3a, Sema3f in vascular cell clusters. (C) Violin plots demonstrating overexpressed growth factors and other markers highly expressed in vascular cell clusters.
Among the many growth factors and other factors, transforming growth factor b family (Tgfb), vascular endothelial growth factor family (Vegf), platelet-derived growth factor a (Pdgfa), fibroblast growth factor 1 (Fgf1), and insulin-like growth factor 1 (Igf1) were upregulated from vascular cell clusters. These growth factors directly or indirectly positively affect the growth (increase in quantity) and regeneration of nerve cells and also regulate the bone remodeling cellular activities (such as recruit-ment, proliferation and differentiation) that affect bone metabolism.
2. Transcriptional changes of vasculogenic markers among nerve cell clusters
Several studies suggest that the bone vascular system plays a significant role in bone remodeling. Bone marrow arteries and capillaries are essential components of bone multicellular units, i.e., sites of bone remodeling [38-40]. The bone vasculature is also presumably important for bone marrow homeostasis, since bone marrow blood vessels play a pivotal role in hematopoietic stem cell niches [41]. Angiogenesis, a physiological process of new blood vessel generation, is one of the regulating processes of bone blood flow and perfusion. Declines in vascular function and angiogenesis are predicted to cause reduced bone volume, bone density, osteoblast activity, and increased osteoclastic resorption [42,43]. This bone loss is also associated with reduced blood flow and nitric oxide synthase impairment [13,17]. Nerve regeneration precedes angiogenesis during bone development, endochondral ossification, bone defect healing, or fracture repair [5,6]. Thus, characterizing the factors secreted by nerves in the regeneration of blood vessels and angiogenesis is required to identify this phenomenon. The transcriptional changes in vasculogenic factors expressed by nerve cell clusters were next identified. Cells were identified as nerve cells (Marker gene: Mpz, Mpb, Sox2, Plp, Sox10) by scRNA-Seq profiling during endochondral ossification. Among the 161 potential angiogenic marker genes, 144 genes were analyzed with scRNA-Seq, and 21 genes were found to be highly expressed and/or upregulated over time in the nerve cell cluster.
These 21 genes included the nerve cell cluster intensely expressed a fibroblast growth factor 1 (Fgf1) gene and neural cell adhesion molecules Nrcam (Figure 2). Fgf1 is a potent angiogenic factor by activating many other angiogenic growth factors, including Vegf, Pdgfr, and hepatocyte growth factor (Hgf), which regulates endothelial cell activation, migration, and vascular maturation [58]. Fgf1 is Vegf-dependent in angiogenesis that contributes to vascular regeneration by activating Vegf [58]. Although scRNA-seq indicated that Vegf was not expressed in neural cell clusters, other transcriptional enrichment results reported that Vegf was generally overexpressed in endothelial cells of bone vessels [51]. Therefore, Fgf1 expressed in nerve cells and Vegf expressed in vascular endothelial cells create synergetic effects and produce angiogenesis that affects bone regeneration. A neural cell adhesion molecule, Nrcam, is important in neural development. Previous research has reported that Nrcam is also expressed in endothelial cells and plays a significant role in endothelial cell tube formation and further angiogenesis [59]. These nerve cell cluster-derived angiogenic factors also control bone remodeling and contribute to the bone formation by supplying appropriate blood flow to a large area of the bone through angiogenesis [44]. Among the 21 genes, other vasculogenic genes, such as Pdgfa, Hbegf, Mydgf, Hspg2, and Ang-ptl4, were upregulated from the nerve cell cluster non-specific.

Relative expression of vasculogenic genes among nerve cell cluster. (A) Representative UMAP visualization of three vascular cell clusters and one nerve cell cluster among 17 different cell clusters. Among the 45 potential neurogenic marker genes, 40 genes were analyzed by single-Cell RNA-Seq. Among the 161 potential angiogenic marker genes, we have analyzed 144 genes with Single-Cell RNA-Seq. Among 144 genes, 21 were highly expressed and most upregulated in the nerve cell cluster over time. (B) Violin plots showing overexpressed vasculogenic gene markers including Fgf1 and Nrcam in nerve cell clusters. (C) Violin plots demonstrating overexpressed growth factors and other markers highly expressed in nerve cell clusters.
In addition to the neurogenic and vasculogenic factors described above, other factors are also thought to affect the neurovascular interaction, and it is believed that continuous follow-up of these factors is necessary. For example, mechanical and systemic hormonal factors stimulate the release of the abovementioned neurogenic and vasculogenic factors, including NGF, semaphorin3a, semaphorin3f, VEGF, and FGF, and ultimately increase bone-associated skeletal nerve fibers and augment blood flow for bone regeneration. Furthermore, there are factors that influence the neurogenesis and vasculogenesis systemically, such as brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), CGRP, and Substance P (SP) promoted by external stress stimulation such as exercise training. More research is needed to elucidate the role of these complex factors.
MODULATION OF PERIPHERAL NERVES AND BONE FOLLOWING EXERCISE TRAINING
Various exercises, especially aerobic exercise, are known to enhance motor nerve function in the event of spinal cord injury or other neurological damage [60,61], and to help with the improvement of cognitive function and attenuation of the diseases such as Parkinson's disease, Alzheimer's disease, and peripheral neuropathy related to diabetes-mellitus and chemotherapy [62-66]. Aerobic exercise is not only involved in the central nervous system but also in promoting neurite extension from the dorsal root ganglia of the peripheral nervous system, which is believed to help regeneration of the entire peripheral nervous system, including the regeneration of axon terminals in the periphery [67]. During a low-in-tensity treadmill aerobic exercise for five days a week for six weeks in mice with median nerve denervation, the amplitude of the evoked motor response increased (p <.05; 61% vs. Non-exercise), and the number, diameter, and myelination of axons were augmented in the median nerve injury site [21]. This suggests aerobic exercise training improved nerve stimulation to the periphery by promoting distal axon outgrowth and inducing better myelination. Likewise, increased nerve regeneration following the sciatic nerve crush was observed following the volunteer wheel running exercise [67]. The mean neural length was increased by 79%, and nerve density measured by labeling neurons was augmented (93%) with voluntary aerobic exercise compared to non-exercise control animals [67]. Increased nerve regenerative capacity following aerobic exercise training was associated with upregulation of neurotrophic factors such as BDNF, neurotrophin 3 (NT3), and Synapsin I in the serum and neuron [21,61,67]. Surprisingly, during exercise conditions, inhibition of the receptor tyrosine kinase (Trk), the receptor for sensory nerve fibers, suppressed the ingrowth of neural length and reduced gene expression of the synaptic vesicle protein, Synapsin I, such that exercise-induced nerve regeneration was no longer observed [67].
Recent research work has also demonstrated that four weeks of treadmill exercise at low intensity (10 m/min/day for 50 minutes) restored the peripheral nerve density in chemotherapy-induced peripheral neuropathy conditions [66]. In addition, paclitaxel-induced peripheral neuropathy significantly disrupted the sensory nerves in the paw skin intraepidermal nerve fibers and provoked thermal hypoalgesia. At the same time, aerobic exercise training recovered the intraepidermal nerve fiber density back to near normal value (∼90% increase with exercise training), such that reduced behavioral and sensory response were no longer observed [66]. Even though it is still unknown the exact molecular mechanisms behind how aerobic exercise interrupts the actions of chemotherapy agents in the axon terminal microtubule dynamics, aerobic exercise training may play an essential role in stimulating neurotrophin factors, improving microtubular stabilization, and promoting nerve length and myelination. Thus, regular physical activities or aerobic exercise training should be considered a reasonable method to recover from diseases and attenuate their impact when considering peripheral neuropathy or peripheral nerve-related pathologies.
Recently, the effect of aerobic exercise training on the volume of trabecular bone and changes in periosteum nerve fibers was reported [19]. Eight weeks of treadmill aerobic exercise training increased trabecular bone volume by a higher trabecular number and augmented the periosteal nerve fiber density [19]. In addition, the authors demonstrated by correlation and microRNA analyses that a significant association between skeletal nerve densities and fractional bone volume of trabeculae was observed, and some of the microRNA, such as miR-29b-3p and miR-491-3p, may be involved in the exercise-derived osteogenic and neurogenic effects [19]. Yet, research on the effectiveness of aerobic exercise on bone regeneration and bone-associated nerves still needs to be improved, the underlying mechanisms still need to be completely char-acterized, and additional experimental works are warranted.
Most of the experimental examinations were based on aerobic exercise (treadmill or wheel running), and the studies on the effects of an-aerobic or resistance exercise on the peripheral nervous system are limit-ed. In the central nervous system aspect, the positive influences of resistance exercise on cognitive function in the clinical population have been documented [68-71]. Although there was no direct measurement of peripheral nerve regenerations, the efficacy of strength and balance training for clinical patients with peripheral neuropathy was recognized, such that the training improved lower body strength and maintained balance, further reducing the risk of falls and fracture [72,73]. Some investigators sought to determine the serum level of neurotrophic factors following resistance training, yet the data have shown mixed outcomes. In one in-vestigation, the serum levels of neuroprotective factor IGF-1 and VEGF were upregulated following 16 weeks of resistance exercise using regular body building machines (hamstring curls, vertical butterflies, leg presses, rowing, bicep curls, and calf raises) with the intensity of 75% of single-repetition maximum (1-RM; the maximal weight the one can lift for the single repetition with correct position) [71]. In an acute exercise study, serum NGF level was significantly increased within 1 hour of post-exercise [74]. However, 15 weeks of resistance exercise showed no changes in BDNF and NGF in the peripheral blood [75]. Thus, given the aggregated data with resistance training effects on peripheral nerves, a clear image did not appear concerning the directions or influences of resistance training on the peripheral nervous system. Nonetheless, more precise and detailed in vivo and in vitro examinations with animal studies are required to support the role of resistance exercise on the peripheral nervous system.
MODULATION OF BLOOD VESSELS AND BONE FOLLOWING EXERCISE TRAINING
Regarding exercise-induced mechanical loading, Wolff's Law described bone adaptation to chronically increased or decreased mechanical stimuli [13,76,77]. Furthermore, exercise-induced mechanical loadings, such as compression and tension, generated pressure gradients that expelled interstitial fluid around bone tissue through the lacunar-cana-licular network [78]. Mechanical force or exercise may increase vascular shear stress in bone microvasculature and blood flow augmentation. Increased blood flow on the bone surface produces shear forces and directly stimulates mechanical-loading-induced activation of bone cellular activities for bone anabolic response [78]. Indirectly, shear stress promotes the release of bone cell stimulating factors, including nitric oxide (NO) and prostaglandins (PGE2) [79,80]. Increased shear force elevated osteocyte activity, as osteocytes are very sensitive to shear stress because of bone mechanical adaptation [81]. The NO and PGE2 support osteoblast differentiation and inhibit osteoclast activity, eventually increasing bone remodeling to reinforce bone gain or maintenance [78]. Thus, the abovementioned processes can explain the bone adaptation caused by regular exercise-induced mechanical loading.
As mentioned, angiogenesis augments blood flow to the bone, and it has been reported that revascularization significantly increased through long-term exercise training, mainly through a VEGF-related mechanism. Previously, Yao et al. reported that treadmill exercise for two weeks increased the vascular density of the proximal metaphysis of the tibia by approximately 20%, accompanied by a significant upregulation of VEGF and VEGF receptor 1 messenger RNA expression [18]. To prove the in-volvement of VEGF in a potential mechanism, Yao et al. blocked VEGF by anti-VEGF treatment for five weeks in conjunction with treadmill exercise and showed a dramatical decrease in bone blood vessel number and a decline in exercise-induced BMD and bone mass gain [18]. These data suggested that vascular adaptation to exercise training is strongly associated with the adaptation to the bone.
Regarding angiogenesis as a means to osteogenesis, zinc-finger tran-scription factor ZEB1, which is mainly localized in CD31 hi endomucin hi blood vessels, has emerged as an angiogenic and osteogenic coupling factor [82]. The blood vessel subtype where CD31 and endomucin are strongly expressed is usually distributed around the growth plate of long bones, and it introduces and transfers oxygen, nutrients, and precursors of bone remodeling cells into the bone, facilitating activation of perivascular stem cells and hematopoietic stem cells, all of which help angiogenesis and osteogenesis coupling [82-84]. This vessel subtype-expressed ZEB1 level is reduced with aging and post-menopause, significantly low-ering the bone blood vessels and further reducing bone mass [82]. Inter-estingly, in a rodent model study, ZEB1-specific deletion from endothelial cells has been shown to inhibit vascularization and osteogenesis in mice, and restoration of ZEB1 resulted in angiogenesis of CD31 hi endomucin hi blood vessels and further bone formation [82]. Furthermore, ZEB1 has been reported to positively affect not only the formation of blood vessels and bone formation but also the differentiation of neurons, promoting the conversion of induced nerve cells to functional nerve cells, and improving nerve pattern formation [85]. Thus, ZEB1 is an important factor that can positively regulate bone angiogenesis and bone-associated skeletal nerve fibers, and influence bone regeneration and remodeling. However, the effect of exercise training on ZEB1 has yet to be established. Therefore, further research is required whether exercise-de-rived ZEB1 can be considered an essential factor when dealing with bone formation and regeneration through nerve-vascular interaction.
Another factor that promotes the angiogenesis of bone blood vessels is CGRP. This factor is normally released from sensory nerve terminals and stimulates new blood vessel sprouting by endothelial cell-residing receptors neurokinin-1 (NK1) and calcitonin gene-related peptide receptor (CGRPR) [86]. CGRP indirectly affects osteogenesis, which can be seen as improving bone vascular function and promoting angiogenesis controlled by CGRP. For example, CGRP improves the vasodilatory function of the principal nutrient artery of the femur in rats in conjunction with inducing bone remodeling and further augmenting bone volume [44]. Furthermore, CGRP promotes angiogenesis by stimulating endothelial cell proliferation and new blood vessel tube formation in a VEGF-dependent manner [87]. CGRP directly promotes osteoprogeni-tor cell proliferation and osteogenic differentiation from MSC, enhancing osteoblast activity [54]. Intriguingly, analysis by RT-PCR and immu-nohistochemistry methods showed a significant increase in CGRP expression in bones after two weeks of moderate-intensity treadmill exercise training, which coincided with increased bone volume in mice [88]. These data suggest that exercise training may be associated with increased expression of peripheral neuropeptides, namely CGRP, which is recognized to play a significant role in angiogenesis and vascular function and osteogenesis potential.
CONCLUSION
The mechanisms of bone-associated skeletal nerves and bone vasculature interaction on bone regeneration are multifaceted, and many genet-ic factors, nerve cell-derived factors, vascular-derived factors, and various cells seem to regulate this interaction. In addition, mechanical stress, such as exercise, recognized as an effective mechanism for bone regeneration, has recently been highlighted as significantly impacting bone-re-lated skeletal nerves, bone vascular function, and angiogenesis in bone. From this perspective, this review paper explored the potential interaction between nerves and blood vessels in regulating bone remodeling and regeneration. It described the effect of exercise training as a mechanical perturbation system on neurovascular coupling in bone (Figure 3). An important question to be addressed is the neurogenic and vasculogenic factors expressed in osteochondral, vascular, and nerve cells, and the stimulation by exercise training and physical activity. These factors may mediate neurovascular interactions and stimulate skeletal nerve fibers and bone blood vessels responsible for positively regulating osteogenesis. Thus, exercise-induced changes in neurogenic and vasculogenic factors may play a significant role in skeletal nerve ingrowth, mediate innervation and angiogenesis, and maintain osteogenic integrity. Explaining neurovascular interactions and the crosstalk with skeletal tissue will enhance the understanding of bone metabolism and pathophysiology of bone-related diseases, including osteoporosis and ectopic bone formation, and improve the characterization of potential disease mitigation and prevention targets. Moreover, studies investigating the effectiveness of exercise training on neurovascular-skeletal coupling would also contribute to developing novel non-pharmacological and non-invasive methods to improve bone metabolism and regeneration. However, more studies are required to understand and detail the underlying mechanisms. This could help developing an appropriate exercise training regimen that targets bone-associated skeletal nerves, bone blood vessels, and bone regeneration all of which could offer new therapeutic methods and solutions to some of the major clinical challenges in the bone field.
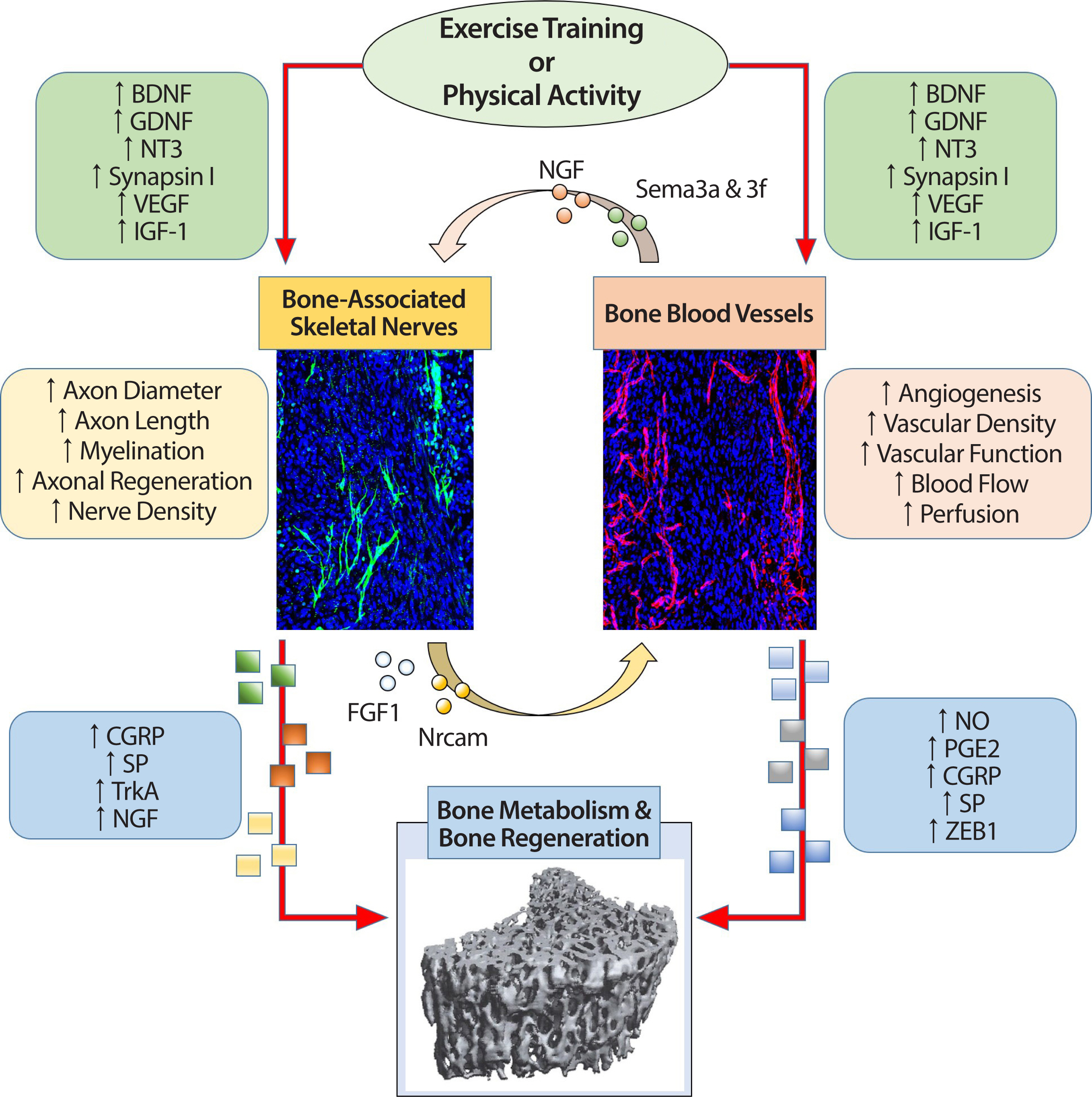
The relationship among exercise training or physical activity, bone-associated skeletal nerves, bone blood vessels, and bone metabolism and regeneration. A schematic view shown in the flowchart describes the potential effects of physiological markers that change through exercise training / physical activity on bone-associated skeletal nerves and bone blood vessels and subsequently influences bone metabolism and bone regeneration. Moreover, this figure, which shows the relationship between each organ, also shows potential physiological markers that enable nerve-vascular interaction and explains the possibility of neurovascular coupling by exercise training/physical activity. The extent to which exercise-induced neurovascular coupling and its role in bone metabolism and regeneration remains a fascinating unreciprocated question.
BDNF, brain-derived neurotrophic factor; GDNF, glial-derived neurotrophic factor; NT3, neurotrophin-3; VEGF, vascular endothelial growth factor; IGF-1, in-sulin-like growth factor-1; CGRP, calcitonin gene-related peptide; SP, substance P; TrkA, tropomyosin receptor kinase A; NGF, nerve growth factor; NO, nitric oxide; PGE2, prostaglandins; ZEB1, zinc-finger transcription factor.
Notes
The author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTION
Conceptualization: S Lee; Data curation: S Lee; Formal analysis: S Lee; Funding acquisition: S Lee; Methodology: S Lee; Project administration: S Lee; Visualization: S Lee; Writing - original draft: S Lee; Writing - review & editing: S Lee.
