Rescue Effect of Exercise on Impaired Arteriolar Myogenic Response with Advancing Age
Article information
Abstract
PURPOSE
Mechanosensitive vascular smooth muscle cells (VSMCs) of resistance arteries crucially regulate blood flow by constricting or dilating over fluctuation of blood pressure (the myogenic response). This review was aimed at introducing cellular signaling that is relevant to arterial myogenic response and briefly describing how the arterial autoregulation is impaired by aging and exercise intervention restores the diminished myogenic responsiveness.
METHODS
A systemic literature research was conducted through PUBMED to comprehend previous studies that explore molecular mechanisms underlying arterial myogenic response, impaired pressure-induced vasoconstriction with advancing age, and effect of exercise training on the arterial autoregulation.
RESULTS
The myogenic response generally consists of three steps: 1) detection of mechanical stress (e.g. stretch, tension) exerted on VSMCs, 2) biological transduction pathways (e.g., depolarization, Ca2+ entry, phosphorylation of myosin light chain, Ca2+ sensitization, actin polymerization), and 3) adjustment of vascular tone (e.g. vasoconstriction or dilation). Aging induces vascular aging that is coupled to increased risks of development of cardiovascular diseases. The intrinsic ability of VSMCs to maintain appropriate blood flow in response to changes in intravascular pressure has been reported to be impaired with advancing age. In contrast, exercise intervention has been demonstrated to rescue aging-induced attenuation of arterial myogenic responsiveness.
CONCLUSIONS
Abnormal myogenic response of resistance arteries leads to vascular rupture, vasospasms, hypertension, or hypotension. Therefore, it will be valuable to investigate the exact mechanisms underlying the contribution of exercise training to arterial myogenic response to prevent and treat impaired arterial autoregulation-induced cardiovascular disorders.
INTRODUCTION
Elevated intravascular pressure increases the radius of blood vessels, which leads to considerable increase in blood flow. Contrarily, decreased intraluminal pressure results in insufficient blood perfusion. As the transitions in intraluminal pressure and subsequent surplus/lack of blood flow cause vascular rupture, edema, or ischemia, small arteries or arterioles (generally < 200 μm of internal diameter) exhibit an intriguing autoregulatory mechanism in response to change in intraluminal pressure to regulate local blood perfusion, minimize capillary hydrostatic pressure, and modulate peripheral vascular resistance [1]. This arterial autoregulation, called the ‘myogenic response’, is defined as intrinsic vascular behavior which elicits vasoconstriction or vasodilation when intraluminal pressure increases or decreases, respectively [2]. The myogenic response is referred to as the inherent properties of vascular smooth muscle cells (VSMCs) since myogenic responsiveness is independent of endothelial cells (ECs) or neurohumoral modulation [1,3]. Abnormal regulation of the myogenic response has been observed in various cardiovascular or metabolic disorders such as subarachnoid hemorrhage, diabetes, congestive heart failure [4]. In this context, as impaired myogenic responsiveness is closely linked to cardiovascular diseases (e.g., hypertension, ischemic stroke, vasospasm), the intrinsic autoregulation has been paid attention as drug targets.
A major culprit of morbidity and mortality in older individuals is largely related to cardiovascular diseases [5]. Advancing age has been demonstrated to cause functional and structural alteration in vascular beds including vascular wall remodeling or excessive vascular stiffness [6]. It has been established along with aging that increased reactive oxygen species (ROS) in the VSMCs and ECs lead to impairment of nitric oxide signaling, increased inflammatory responses, up- or down-regulation of transcriptional factors regulating VSMC proliferation [7]. In contrast to advancing age, physical activity has a plethora of beneficial effects on the cardiovascular system [8]. It has been investigated that exercise training markedly reduces coronary artery diseases, hypertension, and heart failure-mediated morbidity and mortality [9,10]. Thus, exercise intervention may act as a primary or secondary prevention and treatment for cardiovascular diseases. In regard to the arterial autoregulaton that is crucial for the regulation of local blood flow, specific mechanisms by which myogenic responsiveness is impaired with advancing age have not yet fully defined. In addition, whether and how regular physical activity restores the impaired myogenic reactivity in elderly individuals still remains poorly understood. This review has focused on briefly describing intracellular signaling pathways for the myogenic response and how the autoregulation is affected by aging and exercise training.
MOLECULAR MECHANISMS UNDERLYING THE MYOGENIC RESPONSE
For over 100 years, numerous studies in vascular biology have continually sought to investigate intracellular signaling for the myogenic response. Despite these efforts, how the intriguing myogenic responsiveness operates in physiological or pathological circumstances is still unclear. As far as is known, myogenic reactivity is comprised of several processes as follows: mechanosensitive ion channels, receptors, extracellular proteins, and cytoskeletal proteins sense mechanical stresses on vascular wall following changes in intraluminal pressure. The detected mechanical forces are converted to biological signals (i.e., mechanotransduction) such as alteration in membrane potential, Ca2+ influx, myosin light chain phosphorylation-dependent vasoconstriction, Ca2+ sensitization, or cytoskeletal rearrangement for myogenic vasoconstriction [1,4,11].
Increased intraluminal pressure leads the sensory machineries of resistance arteries or arterioles to detect tension or stretch of the VSMCs and then initiate intracellular signaling events for pressure-induced vasoconstriction. Accordingly, the identification of mechanosensors in the VSMCs has been drawing keen attention of leading investigators. To date, integrins and G protein-coupled receptors (GPCRs) have been reported to contribute to detection of change in intraluminal pressure and in turn evoke the myogenic response. Integrins consisting of a non-covalent interaction of α- and β-subunit heterodimers are involved in divergent vasomotor reactivity by regulating Ca2+ dynamics (e.g., extracellular Ca2+ influx) [12]. In addition to the role of integrins in vascular reactivity, it has been elucidated that inhibition of αvβ3 or α5β1 integrin using specific antibodies abolishes myogenic vasoconstriction in skeletal muscle arterioles [13]. Further, more recently, activation of αvβ3 integrin has been delineated to regulate pressure-induced vasoconstriction in cerebral arteries by modulating intracellular Ca2+ waves [14]. Next, since the novel finding that membrane stretch evokes conformational changes in angiotensin II type 1 receptor (AT1R) and provoke downstream signaling in the absence of its ligand, angiotensin II [15], there is growing evidence that deformation of vascular wall following increased intraluminal pressure causes ligand-independent activation of GPCRs in the VSMCs. Specifically, numerous studies have been undertaken to demonstrate using pharmacological inhibition or genetic manipulation (e.g., knockout or knockdown of target GPCRs) that mechanoactivation of purinergic receptors [16], cysteinyl leukotriene 1 receptors [17], and AT1R [18,19] takes part in myogenic vasoconstriction of mesenteric, cerebral, and skeletal muscle arterioles.
Membrane depolarization following an acute elevation in intravascular pressure is a major determinant for myogenic responsiveness of small arteries and arterioles. Transient receptor potential (TRP) channels have been suggested to regulate membrane potential and Ca2+ signaling [20]. Sub-families of TRP channels are comprised of canonical (TRPC), melastatin (TRPM), polycystin (TRPP), and vanilloid (TRPV), akyrin (TRPA), and musolipin (TRPML) channels. Based on biophysical properties of the channels, cations (e.g., Ca2+, K+, Na+) are selectively or non-selectively allowed to be permeable for alteration in membrane potential [20]. With respect to myogenic vasoconstriction, blockade of TRPC6 channel by antisense oligodeoxynucleotides results in significant reductions in membrane potential and myogenic reactivity in pressurized cerebral arteries [21]. Activation of TRPM4 channels being permeable to monovalent cations (e.g., Na+) and regulated by intracellular Ca2+ level and protein kinase C (PKC) has also been identified to induce membrane depolarization and contribute to myogenic reactivity of cerebral arteries [22]. Thus, it is suggested that TRP channels play a critical role in membrane potential regulation and pressure-induced vasoconstriction. Further, epithelial Na+ channel (ENaC) has been thought to be a mechano-gated channels in the VSMCs [23,24]. It is implicated that pressure-induced stretch of the VSMCs may be sensed by extracellular components of ENaC and then the detection may cause the opening of pore-forming components of ENaC [24]. Na+ entry through ENaC is coupled to membrane depolarization and subsequent Ca2+ influx that is required for myogenic responsiveness. Indeed, it has been found that amiloride/benzamil-dependent pharmacological inhibition of ENaC profoundly abolishes myogenic vasoconstriction [25,26].
In contrast to the role of cation entry through TRP channels and ENaC in membrane depolarization following distension of the VSMCs, K+ channels serve as negative feedback regulators that prevent exaggerated myogenic reactivity in resistance arteries. Specifically, the large (big) conductance Ca2+-activated K+ (BKCa) channels and voltage-dependent K+ (Kv) channels participate in modulation of pressure-induced vasoconstriction through K+ efflux-mediated decrease in membrane potential of the VSMCs. Stimulation of BKCa channels by Ca2+ sparks (a vigorous local Ca2+ increase by up to 1-100 μM) exerts spontaneous transient outward currents and in turn hyperpolarization-mediated vasodilation [27]. It has been revealed that increased intraluminal pressure augments the frequency of Ca2+ sparks and knockdown of subunits of BKCa channels markedly increase myogenic responsiveness [28,29]. Along with BKCa channels, Kv channels negatively regulate pressure-induced vasoconstriction. Isolated mesenteric or cerebral arteries treated with selective inhibitors of Kv1, Kv2, or Kv7 channel have shown a significant myogenic vasoconstriction [30,31].
As described above, the activation of diverse ion channels evokes membrane depolarization or hyperpolarization of the VSMCs once intraluminal pressure is altered. Pressure-mediated membrane depolarization has been identified to stimulate voltage-operated Ca2+ channels (VOCCs) [1]. Moreover, inositol trisphosphate (IP3) generated from activation of GPCRs and ryanodine activate IP3 receptors (IP3Rs) and ryanodine receptors (RyRs) in sarcoplasmic reticulum (SR). These events cause Ca2+ release from the SR by activating IP3Rs and RyRs [32]. It has been well-defined that interaction of intracellular Ca2+ with calmodulin (i.e., Ca2+ -calmodulin complex) provokes activation of myosin light chain kinase (MLCK) and MLCK-induced phosphorylation of 20 kDa myosin light chain (LC-20) leads to VSMC contraction by stimulating the MgATPase of actomyosin cross-bridge [33,34]. Thus, Ca2+ influx through VOCCs and Ca2+ release from the SR are key determinants for myogenic vasoconstriction.
However, intracellular Ca2+ signaling in the VSMCs may not be sufficient to account for pressure-induced vasoconstriction since a slight elevation in intracellular Ca2+ has been found in response to increase in intraluminal pressure [35]. Ca2+ sensitization, one of Ca2+-independent mechanisms underlying the myogenic response, is defined as increase or maintenance of vascular contractility in the absence of increased intracellular Ca2+ in the VSMCs. This interesting phenomenon is associated with regulation of myosin light chain phosphatase (MLCP) suppressing the phosphorylation of LC-20 that is essential for VSMC contraction. Myosin phosphatase targeting subunit 1 (MYPT1) and 17 kDa protein kinase C-potentiated inhibitory protein (CPI-17) are phosphorylated by Rhoassociated kinase (ROCK) and PKC, respectively [36,37]. The phosphorylation of those subunits attenuates MLCP activity, which enhances MLCK activity and augments or maintain vascular contractility without a significant increase in Ca2+ level. Suppression of phosphorylation of MLCP considerably reduces myogenic vasoconstriction in cerebral and skeletal muscle arterioles [38]. Next, as for pressure-induced cytoskeletal reorganization, dynamics of actin thin filament has been focused as another Ca2+-independent mechanism underlying the myogenic response. Contractile α-actin filaments have been known to anchor to focal adhesion complexes under integrins embedded in the plasma membrane [39,40]. Actin cytoskeleton reorganization (i.e. transition from globular α-actin to filamentous α-actin) following alteration in intraluminal pressure is obligatory for myogenic reactivity as globular α-actin level is found to decrease along with increase in intraluminal pressure and actin polymerization inhibitors (e.g., cytochalasins, latrunculin) significantly diminish pressure-induced vasoconstriction in cerebral or skeletal muscle arterioles [18,41].
Collectively, the myogenic autoregulation of small arteries and arterioles for the regulation of local flood flow and peripheral resistance is an intriguing outcome of integrity of diverse signaling pathways (Fig. 1).
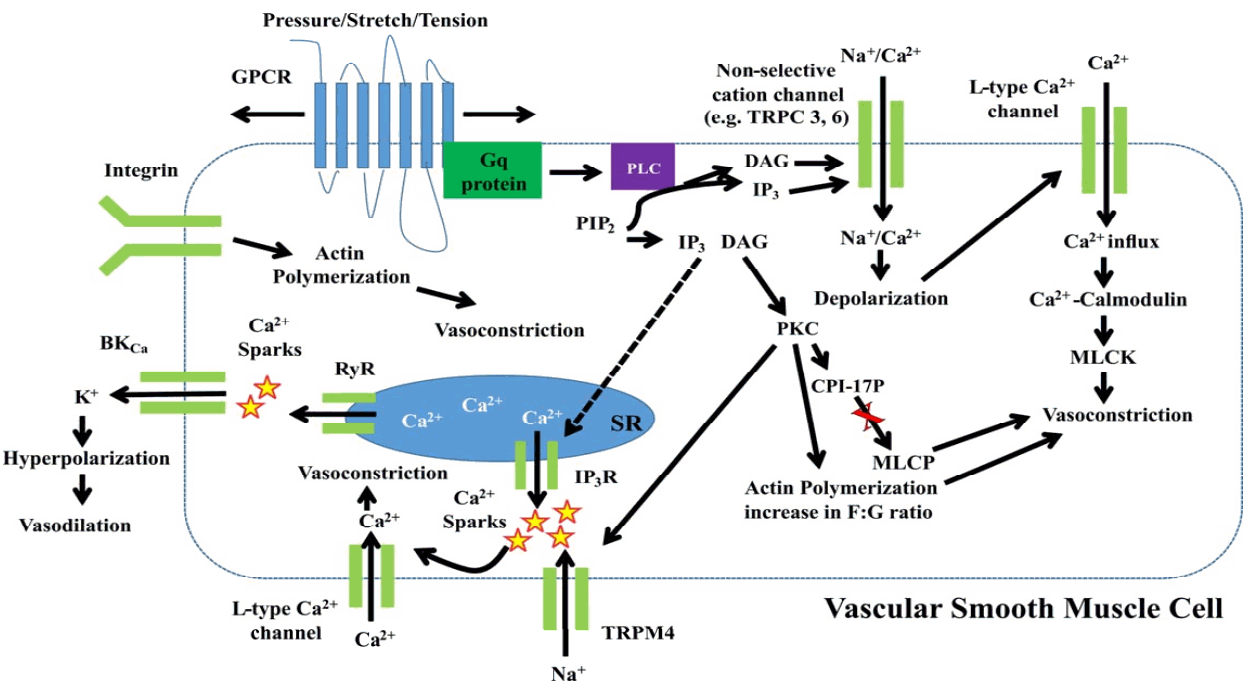
Schematic diagram showing molecular mechanisms underlying arteriolar myogenic vasoconstriction. Abbreviations: BKCa, large conductance Ca2+- activated K+ channel; CPI-17, 17-kDa protein kinase C-potentiated inhibitory protein; DAG, diacylglycerol; GPCR, G protein-coupled receptor; IP3, inositol trisphosphate; IP3R, inositol trisphosphate receptor; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; MYPT1, myosin phosphatase targeting subunit; PIP2, phosphatidylinositol bisphosphate; PKC; protein kinase C; PLC, phospholipase C; RyR, ryanodine receptor, SR, sarcoplasmic reticulum; TRPM4, transient receptor potential melastatin 4.
AGING-MEDIATED ATTENUATION IN THE MYOGENIC RESPONSE
Appropriate blood supply to skeletal muscle is required to perform daily activities and sustained muscle contraction [42]. Impaired physical performance with advancing age may be accompanied by dysregulation of local blood flow and inadequate oxygen supply [42]. Skeletal muscle in old rats has shown a reduction in blood flow capacity following electrical stimulation [43]. Further, it has been demonstrated in human study that elderly individuals have lower leg blood flow and vascular conductance during submaximal-intensity exercise, compared with those of young subjects [44]. In regard to this, a decline in physical performance has been suggested to be due, in part, to age-dependent impairment in myogenic responsiveness [45]. Indeed, myogenic reactivity has been reported to decrease with advancing age in mesenteric, cerebral, and skeletal muscle arterioles [46,47].
Muller-Delp et al. [45] have found that skeletal muscle arterioles isolated from rat soleus muscle (predominantly composed of oxidative muscle fibers) and gastrocnemius muscle (referred to as glycolytic muscle fibers) have a greater myogenic vasoconstriction in young rats compared to those in old rats. The impaired myogenic reactivity with advancing age is not limited to the microcirculation of rodents. Diminished pressure-induced vasoconstriction with aging has been shown in human skeletal muscle arteries [48]. Retinal arteriole autoregulation during exercise (i.e. lifting weights) has been investigated and suggested to be less in elderly individuals [49]. With respect to the decline in myogenic constriction, it has been hypothesized that aging-mediated attenuation of myogenic response is related to alteration in activity of voltage-dependent (Kv) and/or BKCa channels [50]. The activation of K+ channels expressed in the VSMCs typically leads to membrane hyperpolarization that suppresses Ca2+ influx through VOCCs and prevents exaggerated myogenic vasoconstriction [11,31]. Thus, pharmacological inhibition of Kv or BKCa channels with 4-aminopyridine (4-AP) or iberiotoxin, respectively, enhanced myogenic responsiveness in skeletal muscle arterioles in both young and old rats [50]. However, the significant difference in myogenic reactivity of soleus or gastrocnemius muscle arterioles between young and old rats was completely abolished in the presence of 4-AP or iberiotoxin. It is indicated that aging-mediated decline in myogenic vasoconstriction may result from the augmented K+ channel activity in both skeletal muscle arterioles [50].
Beyond small arteries or arterioles in skeletal muscle, the myogenic autoregulation of resistance arterioles in brain such as the circle of Willis and pial vascular bed protects cerebral microcirculation from pressure-induced injury and edema [51]. Even though the importance of the myogenic response has been well-investigated clinically and experimentally [52,53], the question as to how myogenic responsiveness is affected by advanced aging has not fully demonstrated. Middle cerebral arteries isolated from young (3-month-old) and old (24-month-old) mice have shown similar myogenic vasoconstriction in response to increases static intraluminal pressure (i.e., 20-100 mmHg) [47]. However, at the relatively high intravascular pressure (i.e., 140 mmHg), myogenic responsiveness of the cerebral arteries has shown to be significantly lower in old mice, compared to that in young mice. Springo and colleagues [47] applied pulsatile intravascular pressure (pulse pressure frequency: 450/min, pulse pressure amplitude: 40 mmHg) to mimic physiological circumstance. In contrast to static intravascular pressure, it was found that pulsatile pressure-induced vasoconstriction is markedly impaired with advanced age. It is suggested that the inappropriate autoregulation of proximal resistance arterioles causes distal cerebral microcirculation to be exposed high intraluminal pressure and subsequent vascular injury [47]. Previous studies showing the negative effects of advancing age on arterial myogenic response are summarized in Table 1.
Meanwhile, there may be some debate of whether advancing age exclusively diminishes pressure-induced vasoconstriction. There are several subtypes of voltage-dependent Ca2+ channels including L-type, P/Q-type, R-type, and T-type Ca2+ channels in the VSMCs and/or ECs [54]. In contrast to L-type channels, Cav3.2 T-type channels allow local Ca2+ entry in the VSMCs, stimulating BKCa channels and eliciting hyperpolarization-induced decrease in Ca2+ influx and vasodilation [55]. In this context, mouse mesenteric arteries with a deficiency of Cav3.2 T-type channels display augmented pressure-induced vasoconstriction [56] that has been consistently observed with NiCl2, an inhibitor of Cav3.2 T-type channels, in mesenteric arterioles of young mice (8-17 weeks old) [54]. It is indicated that the T-type channels in the VSMCs appear to limit myogenic vasoconstriction. Interestingly, Mikkelsen & Colleagues [54] have demonstrated that the opposite effect of Cav3.2 T-type channels on myogenic vasoconstriction is markedly abrogated in resistance arterioles of adult mice (28-56 weeks old). It is implicated that myogenic autoregulation can be enhanced with advancing age. Thus, further investigation is required for a better understanding of impact of aging on myogenic responsiveness of small arteries and arterioles.
EXERCISE-INDUCED RESTORATION OF IMPAIRED MYOGENIC REACTIVITY WITH ADVANCING AGE
Intrinsic autoregulation of resistance arteries and arterioles in response to changes in blood pressure plays crucial roles in regulation of local blood flow and peripheral resistance in animals and humans [1]. It has been delineated that the ability of resistance arteries and arterioles to modulate vascular contractility for the satisfaction of appropriate local blood perfusion is enhanced by exercise training [57]. To examine intracellular mechanisms by which exercise ameliorates the myogenic response in porcine coronary resistance arteries, PKC-mediated signaling pathways for myogenic vasoconstriction has been paid attention [57]. PKC has been well-established to regulate the myogenic response through L-type Ca2+ channel activation, Ca2+ sensitization, and actin polymerization [38,58]. In this study, coronary arteries isolated from animals involved in a training program (6 miles per hour, 60-minute, 16 weeks) showed greater pressure-induced vasoconstriction than that of control animals [57]. Further, the trained animals exhibited greater attenuation of myogenic vasoconstriction in the presence of PKC inhibitor chelerythrine, suggesting that the enhanced myogenic response in coronary arteries of trained animals is attributed to increased PKC signaling pathways. This is supported by their additional findings showing that PKC-dependent Ca2+ influx through L-type Ca2+ channels was substantially greater in coronary arteries from trained animals. Additionally, decreased Ca2+ entry by PKC inhibition and PKC-α expression at a protein level were considerably higher in the exercise group [57]. Thus, exercise training may enhance the myogenic vasoconstriction of coronary arteries by modulating PKC-related intracellular signaling.
Based on previous work [57], it may be assumed that regular physical activity improves age-related impairment of myogenic response. Indeed, it has been found that treadmill exercise training (15 meter/minute, 15 incline, 20-60 min/day, 5 days/week, 10-12 weeks) consistently enhances myogenic responsiveness in skeletal muscle arterioles isolated from young (4-6 month old) rats and interestingly restores the attenuation of pressure-induced vasoconstriction with advancing age [46]. The restored myogenic vasoconstriction in old (22-24 months old) rats was largely similar to that in young control rats. It has been demonstrated that an increase in Kv channel activity is responsible for a reduction in myogenic vasoconstriction with advancing age [50]. As previously described, K+ efflux through Kv channels leads to membrane hyperpolarization and vasodilation. Thus, it is suggested that aging-induced increase in Kv channel activity diminishes myogenic vasoconstriction of skeletal muscle arterioles in old rats [50]. Consistent with this interesting findings, Ghosh and colleagues have elucidated that exercise training elicits Kv1 channel adaptation (i.e. presumably decreases in channel activity and/or expression), thereby restoring arterial myogenic responsiveness from old rats [46]. However, despite those novel studies [46,57], the mechanisms underlying exercise-mediated enhancement of the myogenic response remain poorly understood. Therefore, further investigations into the role of exercise training in the myogenic response are needed. In addition, it may be worth investigating whether physical inactivity (e.g., bed rest) deteriorates activities of biological machineries that are involved in the myogenic autoregulation and whether the impaired myogenic response by physical inactivity could be restored by exercise training.
CONCLUSION
Small arteries and arterioles are considered as mechanosensitive blood vessels that control their diameter to regulate local microvascular hemodynamics by sensing alteration in intraluminal pressure. Exaggerated or attenuated myogenic responsiveness in resistance arteries has been reported to cause pathophysiological conditions such as hypertension, vasospasm, ischemic stroke, or orthostatic hypotension. The prevalence of vascular diseases is associated with advancing age, which may be partly attributed to age-dependent impairment of myogenic responsiveness in resistance arteries. In view of prevention and treatment of cardiovascular disorders, a decline in the myogenic response with advancing age has been a therapeutic target. Taken together, greater insight of specific mechanisms underlying the impact of regular physical activity on arterial myogenic responsiveness has to be further made to reduce cardiovascular diseases-related mortality in elderly individuals.
Acknowledgements
We cordially thank Dr. Michael Hill, University of Missouri-Columbia, for constructive review of the manuscript prior to submission.
